Signals Recap: Health Policy Shakeup—Medicaid Cuts, Drug Pricing Reform, FDA AI Strategy & CMMI's New Path
Your biweekly collection of news, research, funding & community voices shaping the future of global kidney health
Welcome to one of the busiest months for U.S. health policy, maybe ever. In the span of just a few days, we’ve seen a House GOP proposal that would dramatically cut Medicaid funding (Sunday), a sweeping new executive order on drug pricing (Monday), and launch of CMS Innovation Center’s “Make America Healthy Again” strategy (Tuesday). This week’s deluge follows last week’s leadership changes at two key agencies: Chris Klomp, a former health tech CEO known for his frugal, data-driven approach, is now overseeing Medicare—and Vinay Prasad has officially taken the reins at the FDA’s Center for Biologics. Suffice it to say: this Sunday newsletter became a Tuesday limited edition.
Each of these stories carries big implications—not only for federal spending and pharma policy, but for how millions of Americans access and pay for care.
This week’s recap is another small but necessary step toward the noise. If it’s getting louder, it’s because we’re getting closer to the source. We’re tracking the top policy shifts, regulatory updates, and industry signals, with particular attention to what they mean for kidney care and innovation.
Thank you for being here with us. If you find this recap valuable, consider forwarding it to a colleague or friend who’s working to make kidney health better for all.
In this issue
Trump’s latest Executive Order on drug pricing
House GOP’s proposed Medicaid cuts spark pushback
CMMI unveils its “Make America Healthy Again” strategy
UnitedHealth CEO steps down; 2025 outlook suspended
Vanderbilt adopts portable kidney perfusion tech
FDA names first Chief AI Officer, rolls out AI-assisted reviews
Pegcetacoplan receives Priority Review for rare kidney diseases
Sonavex wins FDA clearance for EchoMap to aid dialysis access
OpenAI launches HealthBench to evaluate medical LLMs
Alport Syndrome Foundation hosts regulatory workshop
Faith Lynch becomes ANNA’s new national president
15 new roles across Medtronic, Novartis, CVS, ADA, and more…
Signals
A wave of policy and regulatory shifts is reshaping the U.S. health landscape—from payment reform and drug pricing pressure to executive shakeups and CMS strategy resets. We’ll unpack many of these in upcoming briefs. For now, here are two major stories we’re watching that will impact the kidney space.
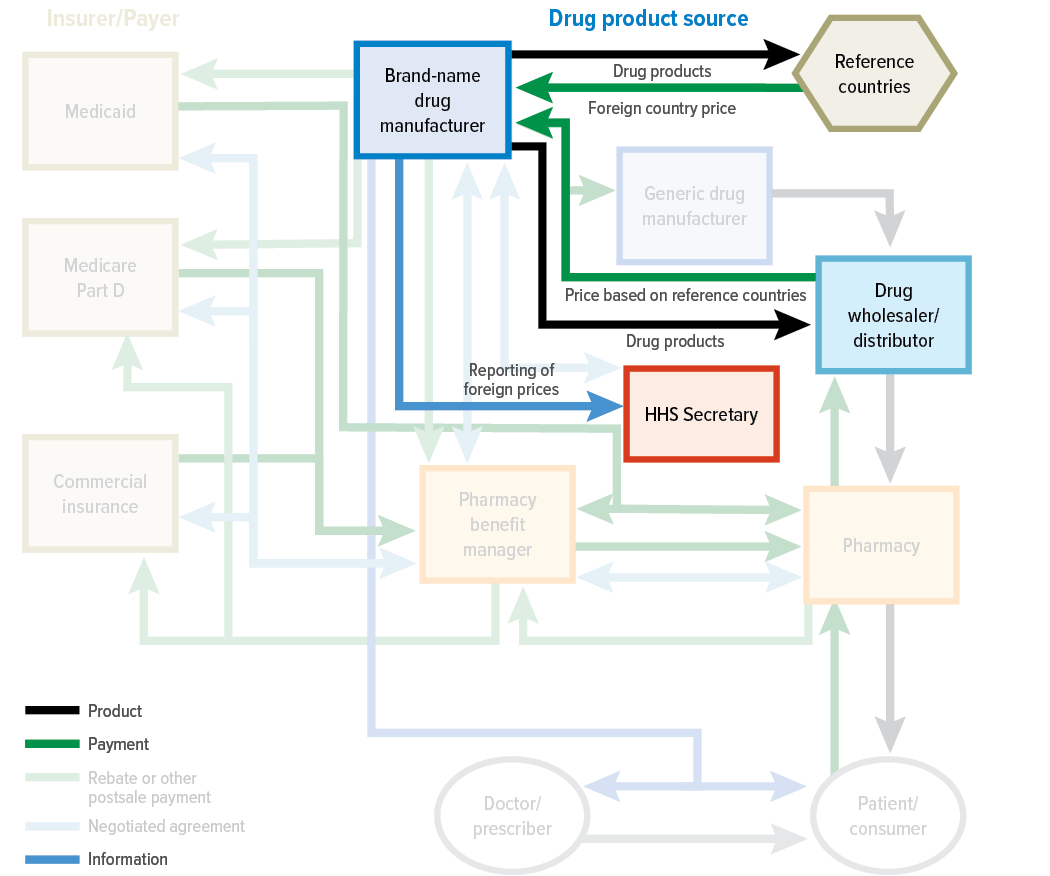
1. Trump’s new executive order revives drug pricing debate—and global pressure on pharma
President Trump has signed an executive order directing HHS to negotiate lower drug prices across public and private markets, with plans to enforce a “most favored nation” (MFN) pricing policy if talks stall. Under the order, HHS has 30 days to set international reference price targets and deliver them to drugmakers, with a proposed rulemaking plan to follow if “significant progress” isn’t made. The policy would cap U.S. prices based on those in peer nations and allow for direct-to-consumer pharmaceutical sales at those rates, while also tasking federal agencies with confronting foreign pricing practices and anti-competitive behavior. The announcement comes amid rising bipartisan momentum to rein in drug costs and follows new legislation from Sens. Josh Hawley and Peter Welch that would penalize companies for charging Americans more than patients in countries like Germany or Canada.1
The pharmaceutical industry is expected to push back, with prior PhRMA statements warning that international reference pricing would undermine investment and risk boosting reliance on foreign suppliers. But pressure is mounting: Americans pay significantly more for prescription drugs than those in other high-income countries—Revlimid, for example, costs nearly $1,000 per pill in the U.S. versus a fraction abroad (and costs 25 cents to make).2 A 2024 Congressional Budget Office report found international reference pricing would yield the greatest cost savings of seven major drug pricing proposals, though it also warned of potential impacts on innovation. Whether this order marks a shift in global drug pricing dynamics—or stalls like its predecessor—will depend on the next round of negotiations and how aggressively HHS moves to implement new rules.34
What do you think? What are you watching when it comes to this conversation and how do you see this impacting pricing and innovation in the kidney space?
2. House GOP proposes $880B in Medicaid cuts, triggering warnings from providers, patient groups, and kidney advocates
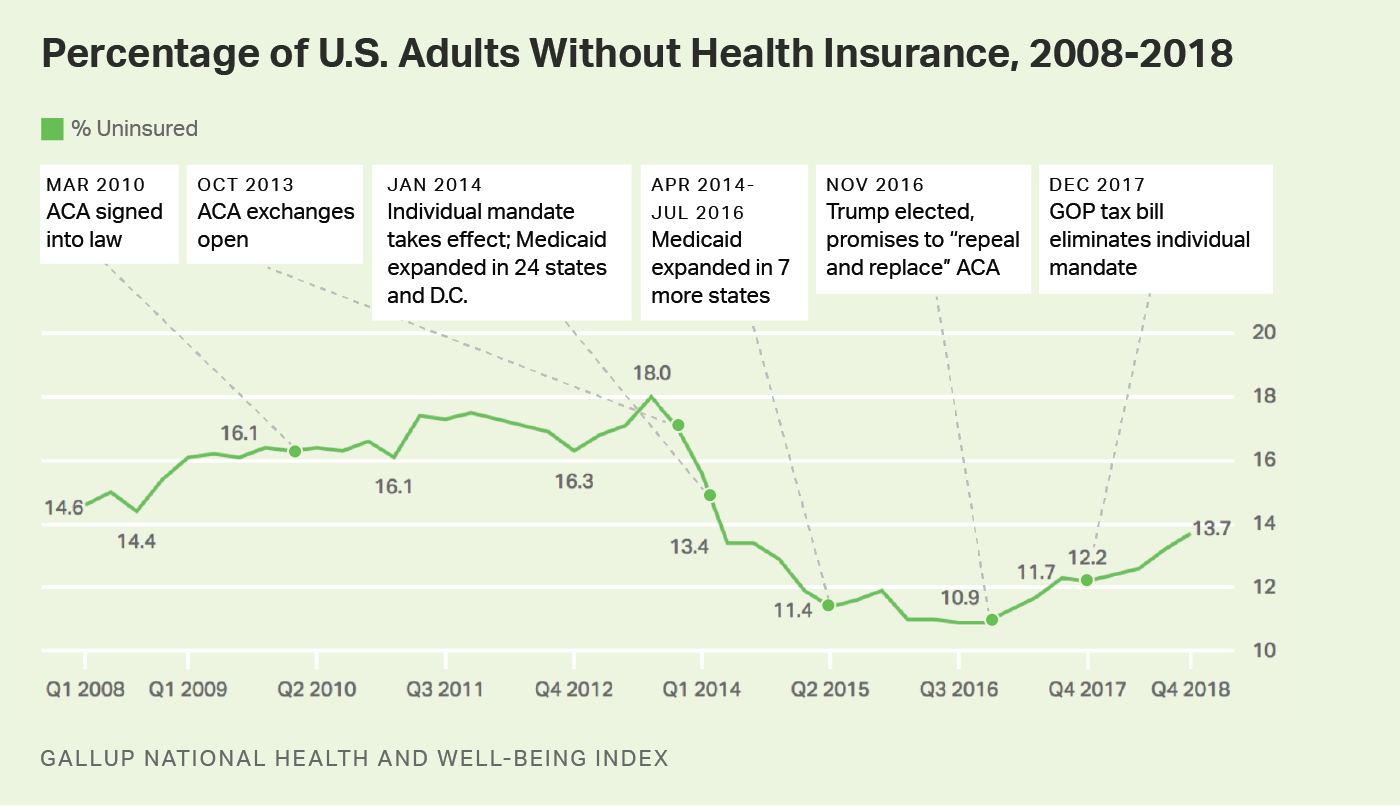
House Republicans have introduced a sweeping budget reconciliation package that would cut an estimated $880 billion in federal spending over the next decade—primarily by tightening eligibility, rolling back ACA provisions, and slashing Medicaid funding. The proposal includes work requirements of 80 hours per month for certain Medicaid enrollees, a shift to biannual eligibility redeterminations, and the elimination of pandemic-era funding boosts. The Congressional Budget Office estimates the changes would leave 13.7 million more Americans uninsured by 2034, with at least $715 billion of the total savings coming from health provisions.5
Industry and advocacy groups have responded with swift condemnation. The National Kidney Foundation warned that the proposals are a “direct threat to the health and survival” of patients, noting that nearly half of all dialysis patients rely on Medicaid. Hospital associations, Medicaid plans, and patient advocacy organizations—including Families USA and the American Hospital Association—echoed concerns that the bill would destabilize safety net programs, reduce access to life-saving care, and push vulnerable Americans into crisis. While Republican lawmakers frame the package as a “common sense” approach to restore fiscal balance, many analysts believe the cuts go too far to gain traction in the Senate. The House Energy & Commerce Committee is expected to hold hearings on the proposal this week as part of a broader push to pass the bill before Memorial Day.6
Visual of the Week
CKD receives $19 per person in research funding. This week’s visual comes from Pranav Garimella, who spotlighted the stark disparity in NIH research investment across major diseases. Despite affecting over 35 million Americans, chronic kidney disease receives only $19 per person—compared to $2,662 for HIV, $2,600 for cystic fibrosis, and $2,370 for sickle cell disease. It’s a timely reminder that research priorities often lag behind the true burden of disease. Personally, at a time when national policy is laser-focused on chronic illness, root causes, and cost containment, I’d hope to see far more—not less—resources directed toward a condition that accounts for 1 in every 5 Medicare dollars.
News
Omada Health files for IPO, signaling renewed momentum in digital health markets. Virtual chronic care provider Omada Health has filed to go public on the Nasdaq under the ticker “OMDA,” marking one of the first digital health IPOs of 2025. With over one million members served and a growing portfolio of condition-specific programs—including GLP-1 support—Omada is positioning itself as a scalable solution for employers and health plans navigating chronic disease management (Fierce, Second Opinion).
FDA grants Priority Review to pegcetacoplan for C3G and IC-MPGN. The FDA has accepted a supplemental New Drug Application for pegcetacoplan (Empaveli) to treat C3 glomerulopathy and IC-MPGN, supported by phase 3 data showing a 68% reduction in proteinuria and stabilization of kidney function. If approved, it would mark the first targeted therapy for these rare, progressive kidney diseases, with a decision expected by July 28, 2025 (Neph Times).
Sonavex secures FDA 510(k) clearance for EchoMap. EchoMap combines 3D ultrasound and AI algorithms to help dialysis technicians visualize fistulas or grafts before cannulation, aiming to reduce complications from missed access. As Dr. Randy Cooper, the study’s principal investigator, noted: “This device offers real promise to reduce cannulation complications and associated fear and stress for our patients.” A post-market study with a large dialysis organization, backed by NIH funding, will evaluate its clinical impact later this year (Press release).
Vanderbilt surgeons first in Tennessee to use next-gen kidney preservation technology. Vanderbilt Health has become the first center in the state to adopt the FDA-approved KidneyVault from Paragonix, a portable hypothermic perfusion system that preserves donor kidneys more safely and consistently during transport. Unlike traditional ice storage, the device provides continuous perfusion and temperature control for up to 24 hours (AHA.org, h/t Paul Gordon).
UnitedHealth CEO Andrew Witty resigns; company suspends 2025 outlook amid continued turmoil. Witty stepped down abruptly Tuesday, citing personal reasons after a turbulent four-year tenure marked by a cyberattack, executive tragedy, and mounting pressure on the Medicare Advantage business. The company also suspended its 2025 profit forecast—just weeks after a 12% downward revision—sending shares down more than 11% in morning trading (Reuters, STAT).
Deerfield Management has raised over $600 million for its third Healthcare Innovations Fund, targeting breakthroughs in therapeutics, care delivery, and AI-enabled technologies. Backed by partnerships with top research institutions and housed at its NYC-based Cure innovation campus, the fund will operationalize innovation through Deerfield’s in-house discovery and development teams (Press Release, Endpoints).
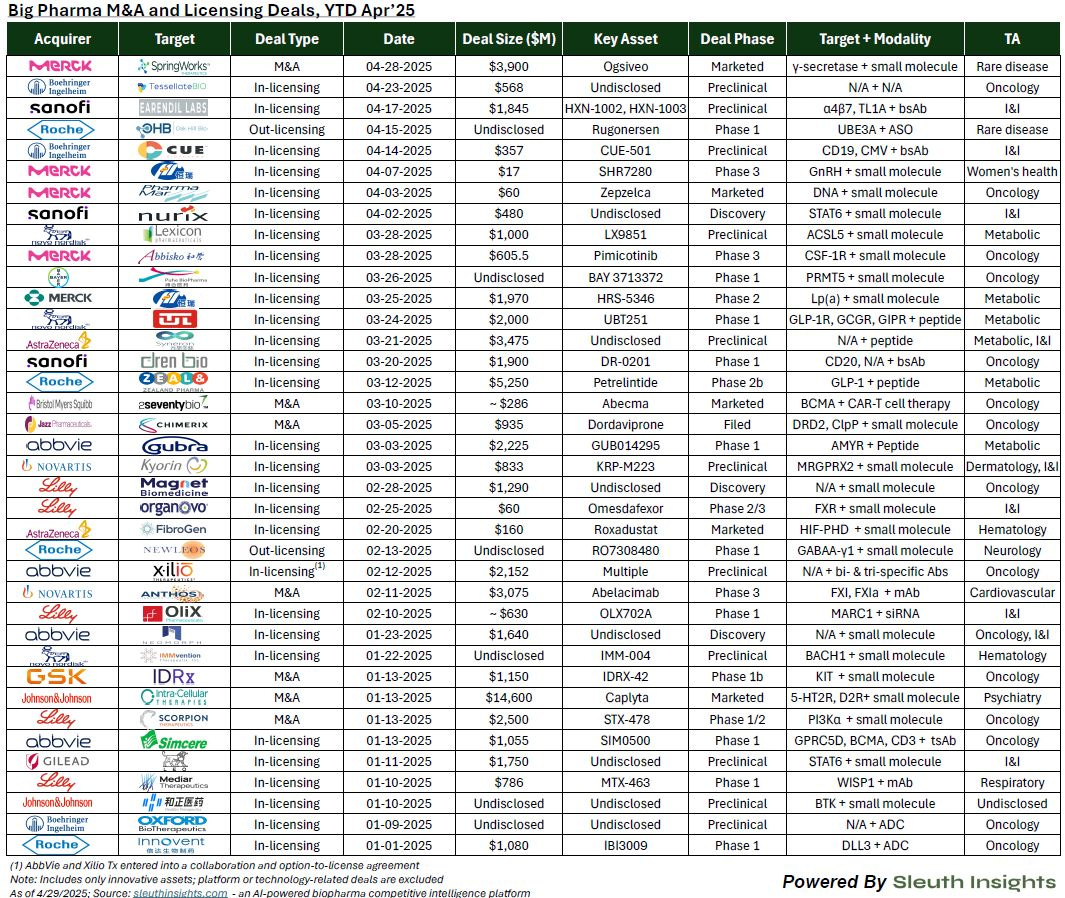
Robust pharma M&A and licensing activity continues despite market headwinds, with $60B in 2025 deal volume. Through early 2025, pharma companies have completed 38 deals totaling nearly $60 billion, with a strong tilt toward early-stage assets—especially in oncology and small molecules. Facing looming patent expirations and attractive biotech valuations, companies like Merck and BMS are doubling down on later-stage licensing and M&A to refill their pipelines (LinkedIn, h/t Andrew Pannu).
Policy & Regulatory
$880B in Medicaid cuts proposed. Republicans have unveiled a sweeping plan to slash Medicaid and ACA spending—introducing work requirements, biannual eligibility checks, and other restrictions—to help offset $4.5 trillion in Trump-era tax cuts set to expire this year. While GOP leaders tout it as a push to reduce fraud and rein in costs, Democrats warn the proposal could strip coverage from over 8 million people, reigniting ACA repeal tensions and raising uncertainty as Trump himself signals opposition to Medicaid cuts (AP News, NYT, The Hill).
Trump administration unveils aggressive drug pricing plan tied to global benchmarks. President Trump announced a sweeping executive order adopting a “most-favored nation” policy that would tie U.S. drug prices to the lowest paid globally and authorize HHS to enforce price targets through federal rulemaking. The order also empowers federal agencies to negotiate directly with industry, challenge foreign pricing policies deemed discriminatory, and expand direct-to-consumer access. While framed as a win for U.S. patients, the administration expects the policy will raise drug prices abroad—bringing new revenue to pharma even as investor confidence wavered, sending stock prices down ahead of the announcement (Reuters, STAT, Axios, The Hill).
CMS Innovation Center unveils “Make America Healthy Again” strategy focused on prevention and patient choice. CMS Administrator Dr. Mehmet Oz and Innovation Center Director Abe Sutton introduced a new strategic direction for CMMI that centers on evidence-based prevention, patient empowerment, and expanded competition across Medicare and Medicaid. The updated framework responds to the Trump administration’s call for transformation and includes plans to scale lifestyle-focused care models, promote digital tools, and build logical payment systems that reward healthier outcomes (CMS.gov, Webinar).
FDA completes first AI-assisted scientific review and sets June 2025 deadline for agency-wide rollout. FDA Commissioner Dr. Marty Makary announced the success of the agency’s first generative AI pilot for scientific review, calling the technology a “game-changer” for reducing repetitive workload and accelerating drug evaluations. The FDA now plans to deploy AI tools across all centers by June 30, 2025, marking a major shift in how therapies are reviewed internally (FDA.gov).
FDA appoints first Chief AI Officer and rolls out generative AI platform. Jeremy Walsh has been named the FDA’s Chief AI Officer as the agency begins deploying a generative AI system to streamline scientific reviews and reduce administrative burden. Walsh brings over a decade of healthcare AI experience from Booz Allen and will lead efforts to modernize FDA operations through cloud and machine learning technologies (MedTech Dive, h/t Ken Nelson).
The Trump administration has appointed former Collective Medical CEO Chris Klomp to lead Medicare. He’s known for his cost-cutting ethos and tech-driven approach, and is expected to prioritize data sharing, efficiency, and digital modernization as he takes the helm of one of the nation’s largest and most expensive health programs. For context, Medicare controls payments and benefits for 68 million older adults and people with disabilities, at a cost of over $1 trillion a year (STAT).
Vinay Prasad named head of FDA biologics center, drawing scrutiny and cautious optimism. Vinay Prasad, a UCSF professor and outspoken critic of the medical establishment, has been appointed to lead the FDA’s Center for Biologics Evaluation and Research (CBER), which regulates vaccines, gene therapies, and the blood supply. While markets reacted with unease—sending the S&P Biotech ETF down over 6%—Prasad’s first speech to FDA staff, described by multiple sources as unexpectedly measured and self-aware, suggested a return to nuance from a figure known as both a rigorous researcher and a polarizing public voice (STAT, MedPage Today, This Week at FDA).
Proposed FDA reorganization could weaken regulatory expertise and disrupt oversight, experts warn. A new JAMA commentary by Drs. Scott Gottlieb and Mark McClellan argues that the DHHS plan to consolidate FDA product centers into a single regulatory office would erode domain-specific expertise and fracture coordination between review and compliance functions. The authors caution that such a sweeping overhaul risks bureaucratic inefficiency and undermining the FDA’s capacity to respond effectively to emerging public health threats (JAMA).
Research
Guatemala-led resolution puts kidney health on the global NCD agenda for the first time. For the first time in history, the World Health Assembly is expected to vote on a resolution to formally recognize chronic kidney disease as a priority non-communicable disease, thanks to a proposal led by Guatemala and backed by 18 countries. The resolution calls for integrated, prevention-focused approaches—including early detection, primary care integration, and equitable access to therapies—to address a global burden that affects 850 million people and disproportionately harms those in low- and middle-income countries (The Lancet).
Ten things I wish I knew as a new peritoneal dialysis nurse. A new guidance from the International Society for Peritoneal Dialysis offers practical advice for nurses entering home PD programs, emphasizing not just procedural skills but also patient education, individualized training, and long-term support. Using a modified Delphi method, experts identified essential insights to help nurses improve patient outcomes and extend time on PD beyond basic clinical competence (ISPD).
New global survey aims to identify barriers in diagnosing and managing pregnancy-associated acute kidney injury (PrAKI). Led by King’s College London and supported by the Global PrAKI Consortium and ISN, this international effort seeks to better understand the challenges frontline providers face in recognizing and treating PrAKI—a leading and often preventable cause of maternal and fetal morbidity worldwide. The 10–15 minute survey is open to all healthcare professionals caring for pregnant or recently postpartum individuals (h/t Liz Lightstone).
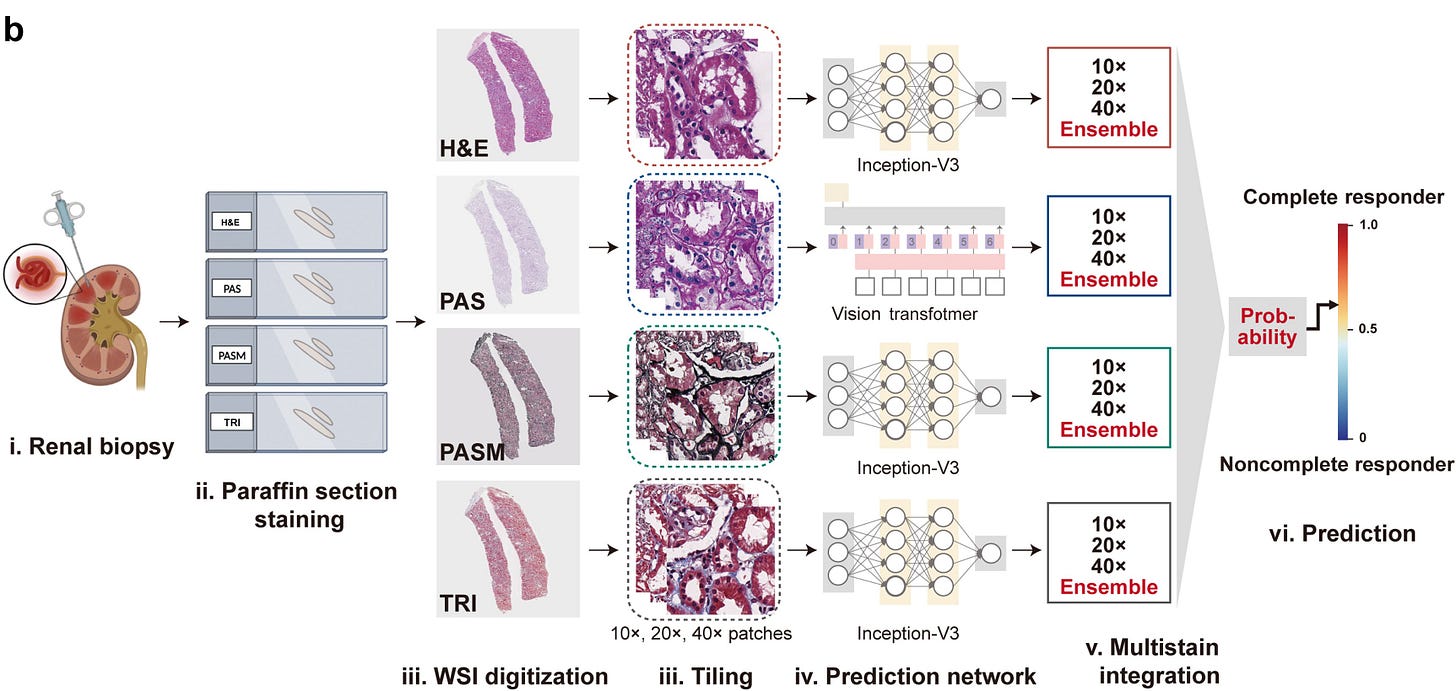
Deep learning model predicts lupus nephritis treatment response from multi-stain kidney biopsies. Researchers developed a deep learning model that integrates multiple renal histopathology stains to predict 12-month treatment response in lupus nephritis, achieving AUCs up to 0.90 in internal validation and outperforming conventional clinical markers. The model identified histologic features such as glomerulosclerosis and interstitial fibrosis as key predictors, offering a potential tool for risk stratification and personalized therapy decisions pending further validation (KI Reports, h/t Davide Garrisi).
Medicare Advantage enrollment linked to lower odds of kidney transplant waitlisting, new study finds. A Regenstrief Institute study published in JASN found that older adults initiating dialysis under Medicare Advantage had an 18% lower likelihood of being waitlisted for transplant within one year compared to those with traditional Medicare—a disparity that persisted after adjusting for health and socioeconomic factors. As enrollment in Medicare Advantage grows, particularly among historically underserved groups, the findings raise concerns that network restrictions and benefit design may be contributing to inequities in transplant access (JASN).
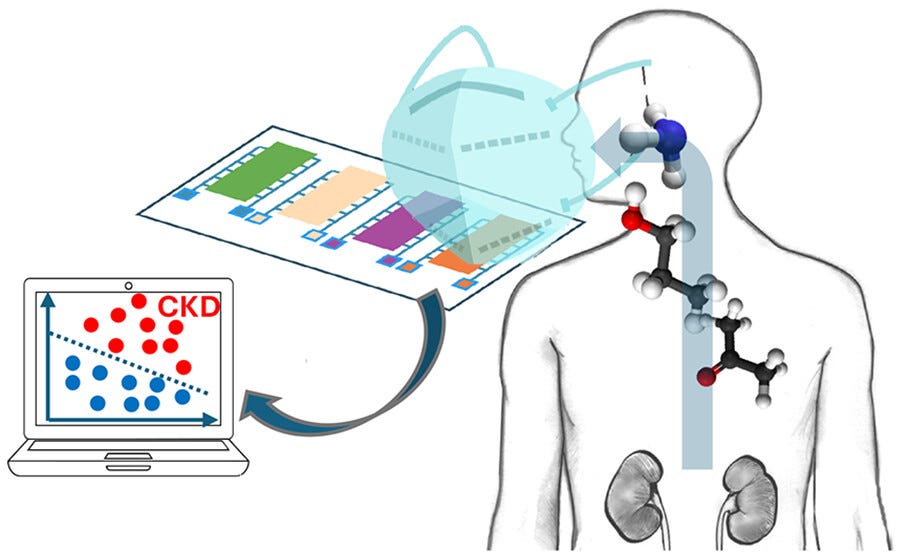
Face mask sensor detects kidney disease through breath with high accuracy, study shows. A new study in ACS Sensors found that a modified surgical mask equipped with a breath sensor could detect chronic kidney disease by identifying elevated ammonia and related gases—correctly diagnosing the condition 84% of the time. Researchers say the technology may help monitor disease progression and support earlier, noninvasive diagnosis in clinical and community settings. (ACS Sensors, see mockup below)
Milken Institute report calls for action to expand connected care for aging at home. A new report from the Milken Institute outlines a roadmap for building a robust connected care ecosystem that supports healthy aging at home through telehealth, remote monitoring, and AgeTech. With 75% of adults over 50 preferring to age in place, the report highlights six core strategies to overcome adoption barriers, workforce gaps, and fragmented care tools—urging coordinated action across health systems, housing, tech, and finance (Milken Institute).
ACOs in Medicare Shared Savings Program reduced costs by up to 2.4% over six years, study finds. A decade-long analysis of over 8 million patients found that ACOs participating in the MSSP lowered per-patient Medicare spending by $294 over six years, with physician-led and smaller ACOs achieving the greatest savings. These results translated to an estimated $4.1 to $8.1 billion in total Medicare savings between 2012 and 2019, underscoring the growing long-term impact of value-based care models (JAMA, see below).
New NEJM AI paper outlines key challenges in applying machine learning to primary care. Unlike hospital data, which is deep and episodic, primary care data is longitudinal, low-signal, and fragmented—making it harder to train effective models. The authors argue that meaningful ML integration will require methods tailored to the realities of primary care delivery, including representative data, whole-person outcomes, and validation strategies for decentralized settings (NEJM, h/t Paulius Mui).
Community
Mark Lim reflected on the recent Alport Syndrome Research & Regulatory Workshop, hosted by the Alport Syndrome Foundation, which brought together families, clinicians, researchers, and FDA and NIH leaders to shape future treatment pathways for this rare genetic kidney disease.
Faith Lynch was officially welcomed as the new National President of the American Nephrology Nurses Association (ANNA), representing over 60,000 nephrology nurses nationwide.
The KARE Initiative, formerly the DECK study, secured local funding to continue its community-based kidney screening work in Richmond, VA (h/t Amber Paulus, PhD, RN).
The American Kidney Fund hosted a Maryland town hall for APOL1-mediated kidney disease awareness, featuring patients, clinicians, and Congressman Glenn Ivey.
Tufts Medicine opened a new Inpatient Abdominal Transplant Unit and hosted healthcare leaders for a tour of its Simulation and Clinical Skills lab (h/t Sharon Klarman).
Carna Health co-founder Boris Berat shared his latest Forbes Technology Council piece on rebuilding healthcare infrastructure with technology that prioritizes real-world complexity over hype.
Christina Farr spotlighted Jacob Reider’s argument for when health startups should buy or build their own EHRs—a nuanced take on product strategy in clinical tech.
Lizzy Lawrence updated STAT’s FDA leadership tracker with recent high-level departures across oncology, drug marketing, and device divisions.
Sachin Jain, MD, MBA reminded us that many of today’s “new” ideas—like value-based care and social services integration—are deeply rooted in earlier models, and still evolving.
Raihan Faroqui, MD recapped the NAACOS Spring Conference, where stakeholders debated AI use cases, MSSP reforms, and operational challenges in managing mixed Medicare panels.
Anna Kaltenboeck raised a timely question around a new ProPublica piece: what threshold of promise should justify investment in new drug development—and how do we define “enough” in today’s R&D environment?
A new primary care training course from XPC is now freely available, offering 15 sessions on clinical workflows, payments, and operations (h/t Paulius Mui, MD and Kenneth Qiu, MD).
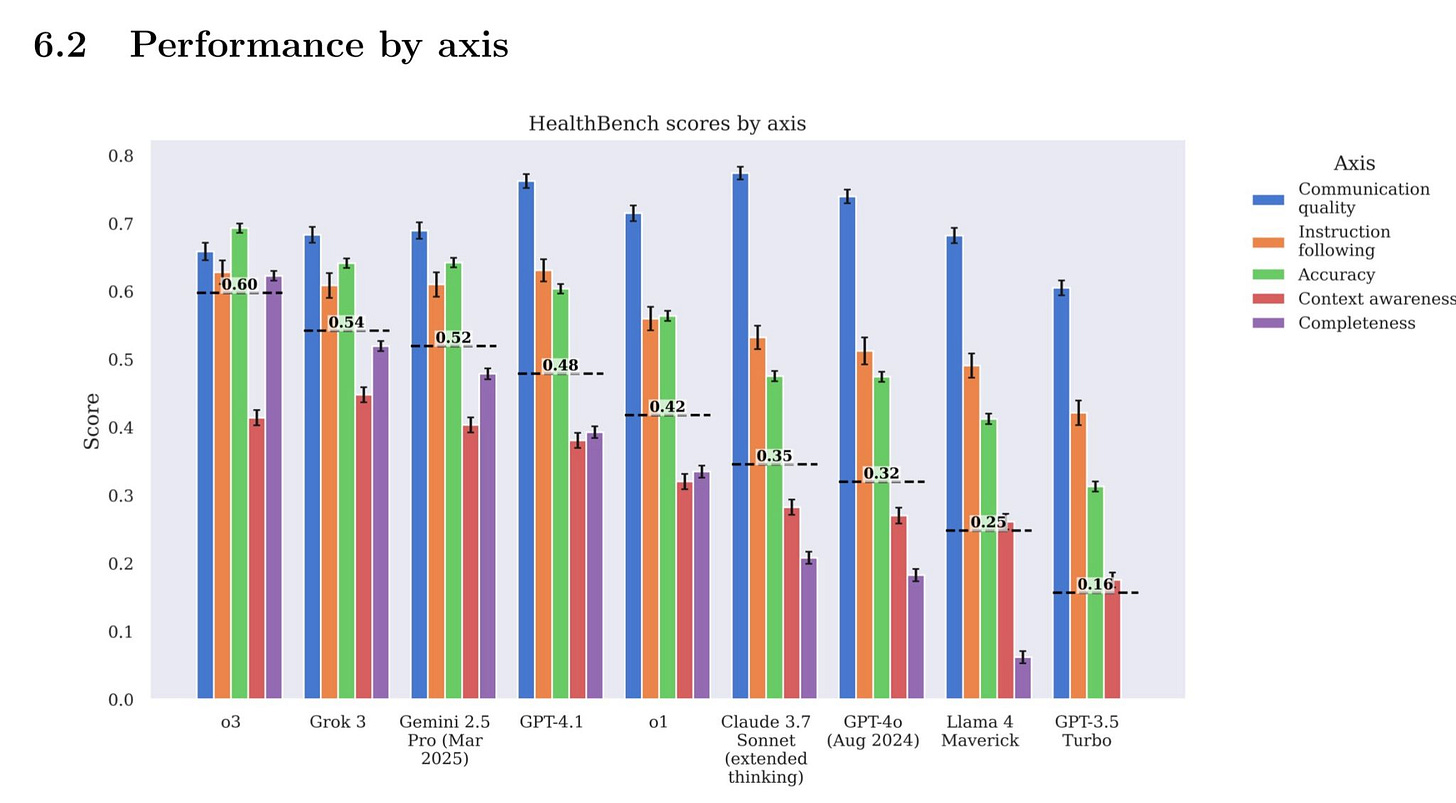
Jonathan Slotkin highlighted the launch of HealthBench, OpenAI’s new benchmark to evaluate healthcare LLMs using 5,000 multilingual case conversations contributed by 262 physicians in 60 countries (see image).
Events Calendar
UCSD Reimagine Grand Rounds (May 14 in San Diego, CA)
VASA 2025 (May 16-17 in St. Louis, MI)
Kidney Innovation Conference (May 21-22 in Washington, DC)
AMGA Value Roundtable (June 9-10 in Washington, DC)
Benesch Nephrology & Dialysis Conf (June 26 in Chicago, IL)
Jobs
Sr Director Strategy and Transformation at Medtronic
Principle Market Development Specialist - RDN at Medtronic
Organ Health Specialist (Brooklyn) at Natera
Global Program Clinical Head - Renal at Novartis
Sr. Patient Relations Manager at Oak Street Health
Principal – Evidence & Strategy at Avalere Health
Global Head, Rare Disease Development at Recursion
VP & CISO at Boston Children's Hospital
Account Sales Representative IV at Mozarc Medical
AVP, General Manager - Medicare (NY/NJ) at CVS Health
APAC Pricing Analyst at Mozarc Medical
Vice President, Information Technology at Akebia
Head of Industry, Healthcare at Viant Technology
Director of Impact and Innovation at Novartis Foundation
Advocacy Coordinator at American Diabetes Association
Senior Director, Policy and Strategy at American Diabetes Association
Associate Professor/Clinical Investigator - Nephrology at Geisinger
Browse 50+ more at jobs.signalsfs.com…
Moving You Forward
Thank you for reading Signals, the leading newsletter for kidney innovation, ideas, and investment. We recently launched Signals Advisory to help companies, investors, and organizations navigate the evolving kidney research, regulatory, and commercial landscapes. Share your next milestones here.
Why do Americans pay more for prescription drugs? (propublica.org)
CBO Report: Alternative Approaches to Reducing Prescription Drug Prices (cbo.gov, October 2024)
![Signals From [Space]](https://substackcdn.com/image/fetch/$s_!IXc-!,w_40,h_40,c_fill,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F9f7142a0-6602-495d-ab65-0e4c98cc67d4_450x450.png)
![Signals From [Space]](https://substackcdn.com/image/fetch/$s_!lBsj!,e_trim:10:white/e_trim:10:transparent/h_48,c_limit,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F0e0f61bc-e3f5-4f03-9c6e-5ca5da1fa095_1848x352.png)