Signals Recap: System Under Review—HHS, CMS & OPTN in Transition, Avalere's Latest CMMI Analysis, & More...
Your biweekly collection of news, research, funding & community voices shaping the future of global kidney health
It’s been a busy couple of weeks in kidney care and beyond. After back-to-back meetings at RPA and NKF, we’re still catching up with all of you and everything happening across the field. Thanks to everyone who stopped by to share what’s on your mind and what’s changing in your corner of kidney health.
In this issue, we’re digging into the two biggest headlines: sweeping HHS layoffs and CMS leadership changes, and the mass resignation of patient representatives from the OPTN Board. We also highlight dozens of headlines, research updates, and community voices that are shaping the kidneyverse right now.
We’re also excited to officially launch two major new efforts at Signals:
Our Kidney Jobs site, where you’ll find some of the most compelling roles and key hires in the space. We connect bright minds with bold teams—try it out, and share any feedback or high-priority roles you’d like to see featured.
Our Expert Advisory Network, which brings together subject matter experts across research, policy, industry, and care delivery to help organizations navigate the kidney landscape with confidence. Get matched for virtual 1:1s and roundtables on topics like discovery, pricing, market access, or value-based care.
Big welcome and thank you to our newest partners, Natera and Guaranteed, and the eight amazing teams who’ve signed on already this month. Your support makes this work possible.
As always, thanks for being here. Let’s keep exploring.
In this issue
HHS shakeup and CMS overhaul raise big questions for kidney care
Patient reps resign from OPTN Board over lack of voice
Avalere: CMMI models cost billions—but some show promise
FDA approves new IgAN drug, expanding treatment options
Pig kidney explant sets xenotransplantation milestone
Interwell CEO: “You have to do everything. That’s what makes it hard.”
Research on glomerular disease, home dialysis, ischemia & more
Community updates from NKF, GloPAKH, Vitality, & more
20+ new open roles now live on jobs.signalsfs.com
This edition of Signals is brought to you by Natera. With over 130,000 patients tested with the Renasight™ test, Natera has the largest commercially available kidney disease database that can help accelerate research and drug development in the space. Discover how Natera’s Renasight™ database helped accelerate an Alport Syndrome clinical trial by 3x. Thanks team!
Signals
What’s happening at HHS?
Layoffs, leadership shifts, and contradictory signals on chronic disease are reshaping federal health priorities—and raising big questions for kidneys.
Summary: The US Department of Health and Human Services is undergoing its most dramatic transformation in decades. Following a March executive order, HHS Secretary Robert F. Kennedy Jr. announced the elimination of 20,000 federal health jobs and a reorganization consolidating 28 offices into a new Administration for a Healthy America (AHA). Newly confirmed Dr. Mehmet Oz will lead CMS. While Kennedy says the goal is to “end the chronic disease epidemic,” the scale and pace of change have sparked concern among researchers, regulators, and providers.
Thoughts: Entire teams at FDA, CDC, and NIH have been cut, including units responsible for tobacco control, infection surveillance, and medical countermeasures. 3,500 FDA staff were part of the first wave of layoffs. Former FDA Commissioner Robert Califf warned that institutional knowledge is being lost “overnight.” Meanwhile, the Center for Medicare and Medicaid Innovation (CMMI), which oversees payment reform pilots like the now-terminated ETC Model, is facing renewed scrutiny. A new report from Avalere Health found that while CMMI lost $6.4 billion across 18 models, several—including disease-specific efforts—showed promise in reducing costs and improving care. Critics worry that ending models early may undermine longer-term kidney care innovation.
The kidney community is watching closely. In February, Dr. Jesse Roach of the National Kidney Foundation called the layoffs “haphazard and indiscriminate,” highlighting direct impacts on transplant oversight and dialysis safety. Kennedy had pledged to appear quarterly before the Senate HELP Committee but skipped a scheduled hearing last week. Meanwhile, the FDA’s top vaccine official, Dr. Peter Marks, resigned citing disagreements with Kennedy. HHS has acknowledged that 20% of the recent 10,000 job cuts may have been issued in error.
Those contradictions deepened after the sudden termination of the NIH’s Diabetes Prevention Program, a 25-year research effort involving 27 institutions. Though the program helped define how to prevent and manage one of the nation’s most expensive and deadly chronic conditions, it was defunded—seemingly not due to merit, but because its principal investigator had shifted affiliations to Columbia University, which recently lost $400 million in federal contracts. “This is a huge mistake,” said Dr. Ezekiel Emanuel, warning that dismantling high-impact, multi-institutional research in the name of politics could undermine the very priorities HHS claims to champion.
What do you think? Will this restructuring and renewed focus on chronic disease be the tailwind kidney care needs to move forward? Or will the disruption and uncertainty stall momentum across payment reform, reimbursement, and innovation?
Patient Leaders Resign from OPTN Board Amid Growing Frustrations
Summary: Last week, nearly all patient and donor family representatives on the OPTN Board of Directors resigned in protest, citing a lack of voice in major decisions tied to the ongoing OPTN Modernization Initiative. In their joint resignation letter, the group said they could “no longer, in good conscience, participate in a Board where our voices go unheard.”
Thoughts: Their departure comes at a pivotal time, as HRSA works to implement recent legislation passed by Congress to reform the U.S. transplant system. In a February letter to HHS Secretary RFK Jr., Senators Bernie Sanders and Ron Wyden urged the administration to continue these efforts, noting that breaking up the OPTN contract—long held by UNOS—was “necessary” to achieve the goals of the 2023 Securing the U.S. OPTN Act.1
The resignations have prompted a wide range of responses. AOPO and ASTS both released statements acknowledging the concerns raised by the patient and donor family representatives, and expressing the need for careful reflection as HRSA moves forward (here and here). Amid strong feelings on all sides, voices in the community are calling for a more empathetic, inclusive dialogue. As Miriam Godwin shared, “There can be no higher stakes issue for policy and oversight than the allocation of donated organs… We will be better served by treating one another as human as we try to create a better system for patients.”2
What do you think? As the U.S. transplant system evolves, what does meaningful patient representation look like—and where do we go from here?
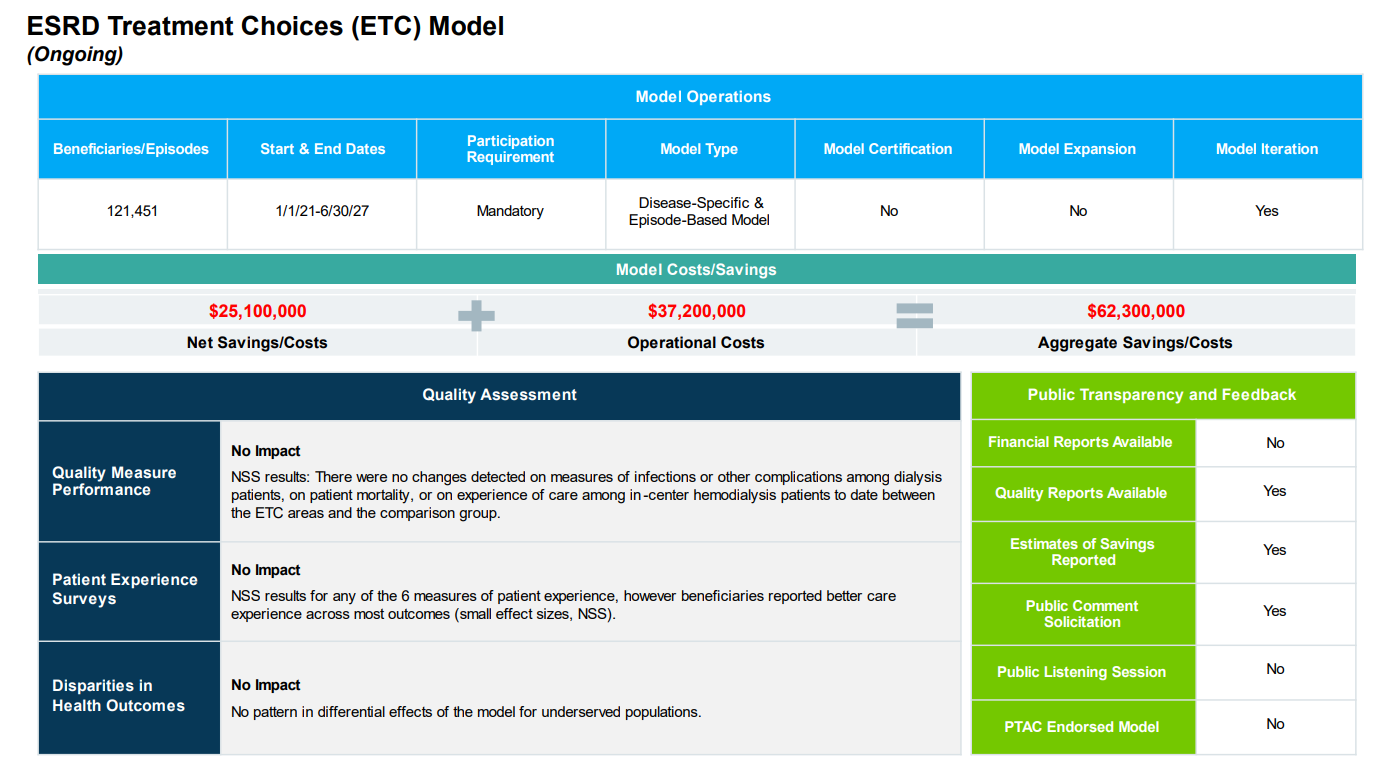
Visual of the Week
A Closer Look at the ETC Model. New analysis by Avalere Health sheds light on the complex financial impact of CMMI's payment models. The ESRD Treatment Choices (ETC) Model—designed to boost home dialysis and transplant rates—ended up costing Medicare over $62 million, with limited quality improvements to show for it. But the report doesn’t just highlight failures—it offers a nuanced view of why many models fall short. Voluntary models may suffer from selection bias, while mandatory models like ETC risk unintended consequences without smaller-scale pilots. Still, one-third of all CMMI models did achieve meaningful savings, and the report says CMMI will likely continue to refine its model portfolio and development process.
Looking ahead, changes in agency leadership and policy priorities—especially under the newly formed Administration for a Healthy America—could reshape how models are designed, tested, and scaled. For kidney care, all eyes now turn to the future of Kidney Care Choices (KCC), IOTA, and the continued rise of Medicare Advantage, as CMS recalibrates its strategy on payment innovation.
News

Ms. Towana Looney, a 53-year-old Alabama woman, lived with a genetically modified pig kidney for a record 130 days before doctors at NYU Langone removed the organ due to signs of acute rejection. While she has returned to dialysis, her case marks the longest survival of a xenotransplant recipient to date. Dr. Robert Montgomery said the outcome is “not a setback” for the field, emphasizing the progress made in extending graft viability (NYT, STAT, NPR).
A team at Massachusetts General Hospital successfully transplanted a kidney that had been frozen for 10 days, marking the first time a cryopreserved organ was transplanted into a large animal. Some say the advance could eventually extend kidney storage times beyond 36 hours, easing the organ shortage and enabling elective transplants (NYT).
Novartis announced a $23 billion investment to build and expand 10 U.S. manufacturing and R&D sites, citing a U.S.-first strategy and pressure from looming pharmaceutical import tariffs. Two new plants for cancer therapies will be located in Florida and Texas, with more than 5,000 jobs expected across operations and construction (Reuters).
The FDA granted accelerated approval to Vanrafia (atrasentan), a new treatment from Novartis for adults with IgA Nephropathy (IgAN) at risk of rapid disease progression. Based on interim data from the Phase 3 ALIGN trial, Vanrafia reduced proteinuria by 36.1% and showed a favorable safety profile. It's the third US approval for Novartis for its kidney disease portfolio in the last year, and the fourth FDA-approved therapy for IgAN, marking a significant expansion in treatment options for this rare kidney disease (NephCure).

Interwell Health CEO Robert Sepucha says he’s unfazed by policy turmoil in D.C.—the real challenge came after merging three companies into one. In a recent interview, he emphasized that winning in kidney care requires more than home dialysis or analytics alone: “You have to do everything. That’s what makes it hard.” H/t Gabriel Perna, Modern Healthcare (paywalled)
The Trump administration will increase Medicare Advantage payments by 5.06% in 2026—an additional $25 billion over last year’s rate. The move provides relief to insurers like Humana and CVS Health, which had struggled with rising medical costs, but experts caution it may not translate to richer benefits for enrollees. Healthcare costs remain high, and hospitals may still shift the financial burden to employers and retirees (Barron’s).
As HHS Secretary RFK Jr. promotes a national push to prevent chronic disease, two major NIH-backed programs focused on diabetes, kidney disease, and dementia have been quietly discontinued. One of them—the Diabetes Prevention Program, a 25-year research initiative—was terminated after its grant was routed through Columbia University, which lost $400M in federal funding amid political controversy. Researchers warn the cuts threaten progress in areas Kennedy has publicly prioritized (NYT, STAT).
The Clinical Accelerator is partnering with Proton Intelligence to launch a feasibility study on continuous potassium monitoring in dialysis patients. Led by Dr. Marat Fudim at Duke University, the study aims to explore novel tech-based approaches to preventing hyperkalemia and hypokalemia in people with CKD and CHF (Clinical Accelerator).
The FDA approved UPLIZNA (inebilizumab-cdon) as the first and only treatment for adults with IgG4-related disease (IgG4-RD), a rare and chronic immune-mediated condition. Developed by Amgen, UPLIZNA reduces disease flares and dependence on long-term steroid use. The approval marks a major milestone for patients living with this complex, multi-organ inflammatory disease (Press release).
Research
GlomCon Hawaii 2024, the inaugural international hybrid conference on glomerular diseases, brought together 619 participants and a faculty of 52 experts for four days of cutting-edge discussion on pathogenesis, clinical management, and trials. With over 26 million social impressions, the event reflected growing momentum in glomerular disease research and a renewed multidisciplinary focus on treatment innovation and collaboration (Glomerular Diseases).
A new Health Affairs piece argues that regulatory failures—not just market dynamics—have crippled innovation and quality in dialysis care. The authors point to burdensome quality programs, certificate-of-need laws, and network adequacy requirements that entrench incumbents, inflate prices, and stifle new entrants. They call for CMS to simplify rules, eliminate loopholes, and expand flexibility models like Kidney Care Choices. H/t Eugene Lin, Ge Bai, and Erin Trish
New data and clinician insights shared at the Annual Dialysis Conference suggest that solo home hemodialysis (HHD)—once seen as risky—is already happening more than many realize. A 2017 FDA clearance allowed unassisted use of the NxStage System One, but surveys show that patients were already going solo before formal approval. While some clinicians remain cautious, experts like Dr. Osama El Shamy argue that supporting safe solo HHD can expand access for motivated patients (Healio)
A study from Children’s Hospital of Philadelphia found that only 22% of families who preferred a language other than English received a living donor kidney for their child, compared to 73% of English-speaking families. Presented by Dr. Stephanie Kerkvliet at NKF’s Spring Clinical Meetings, the study identified unique linguistic, social, and logistical barriers to pediatric living donation—including transportation access, interpreter use, and insurance status (Healio).
A new case series highlights the importance of genetic testing and multi-disciplinary evaluation for kidney transplant candidates with hereditary transthyretin (TTR) amyloidosis. Two Black women with end-stage kidney disease and the Val142Ile TTR variant were cleared for transplant after cardiac amyloidosis was ruled out. The study underscores the need for integrated nephrology, cardiology, and genetics collaboration in transplant evaluation. (AJT, h/t Yasar Caliskan).
At NKF’s Spring Clinical Meetings, Dr. Niloufar Ebrahimi presented two case-based posters: one detailing the successful use of delayed-release budesonide in managing IgA nephropathy in a patient with chronic hepatitis B, and another exploring progressive lymphadenopathy that revealed IgG4-related disease as the underlying cause of kidney dysfunction. Both cases underscore the diagnostic complexity and treatment nuance in glomerular disease (LinkedIn)
A new case report describes idiopathic nodular glomerulosclerosis (ING) in a non-smoking patient with long-term passive smoke exposure and newly diagnosed hypertension. The 60-year-old woman, who also had POEMS syndrome, was diagnosed after biopsy findings showed nodular mesangial expansion. The case underscores passive smoking as a potential risk factor for ING, even in the absence of direct tobacco use (JIMHICR, h/t Sayna Norouzi)
A new study challenges long-held assumptions about ischemic time in heart transplantation, suggesting that cold storage is far from passive. Researchers mapped molecular changes during preservation and found that hearts stored up to 260 minutes showed no cases of severe graft dysfunction. The paper calls for a shift away from the “colder is better” mindset—arguing for cell-type-specific strategies, updated preservation solutions, and a deeper understanding of how molecular stress impacts organ viability. This isn’t the end of the ischemic time debate—but, as the authors put it, “the beginning of the beginning.” (JHLT)
Community
The Global Patient Alliance for Kidney Health’s Steering Committee wrapped up a two-day meeting in Boston last week, focusing on the global burden of chronic kidney disease (CKD) and strategies to elevate awareness through advocacy and collaboration. GloPAKH’s Medical Advisory Council continues to guide its efforts by identifying unmet clinical needs and shaping policy-focused solutions (LinkedIn).
The NKF Innovation Fund is addressing a long-standing gap in funding for kidney and transplant startups. With 1 in 5 donated kidneys currently going unused due to damage or transportation delays, NKF is investing in technologies that improve preservation and delivery—including advanced cold storage, normothermic machine perfusion, and real-time GPS tracking (NKF).
The ASN’s KidneyCure program saw a near-record 155 applications this year for its postdoctoral and independent investigator awards, aimed at fueling the next generation of kidney research. The Grants Review Committee, chaired by Leslie Gewin and Jeffrey Schelling, convened earlier this month to review submissions with support from Zach Cahill and Jennifer Kang. Since 2012, KidneyCure has funded early-career scientists across basic, clinical, and translational research—filling a critical gap in the research pipeline (LinkedIn, h/t Mark David Lim).
ER doc Graham Walker sparked conversation with his comment that “The Pitt” star Noah Wyle is saying more about US healthcare than many leaders and policymakers—highlighting the power of narrative in healthcare reform (Variety)
Mayo Clinic released a new podcast episode exploring pancreas transplantation, surgical innovation, and care coordination models (Mayo Clinic, h/t Andrea Thorp).
Adam Wilson, founder of Vitality, called out the disconnect between national dialysis conferences and real change in clinics—arguing that most frontline nurses and techs are left out of the decision-making process. “If we can drive better outcomes with a small team, others can too,” he said, pointing to Vitality’s success with home dialysis, reduced hospitalizations, and improved patient quality of life (LinkedIn, h/t Adam & team).
At NKF’s Spring Clinical Meetings in Boston, Karin Hehenberger raised a crucial question: Where are the patient voices? While the event brought together researchers, providers, and industry leaders, Dr. Hehenberger noted the noticeable absence of patient perspectives in clinical and scientific discussions. “Inclusion is key,” she wrote—emphasizing that only by inviting patients to the table can we build a more authentic, impactful approach to kidney advocacy and research (LinkedIn, h/t Karin Hehenberger)
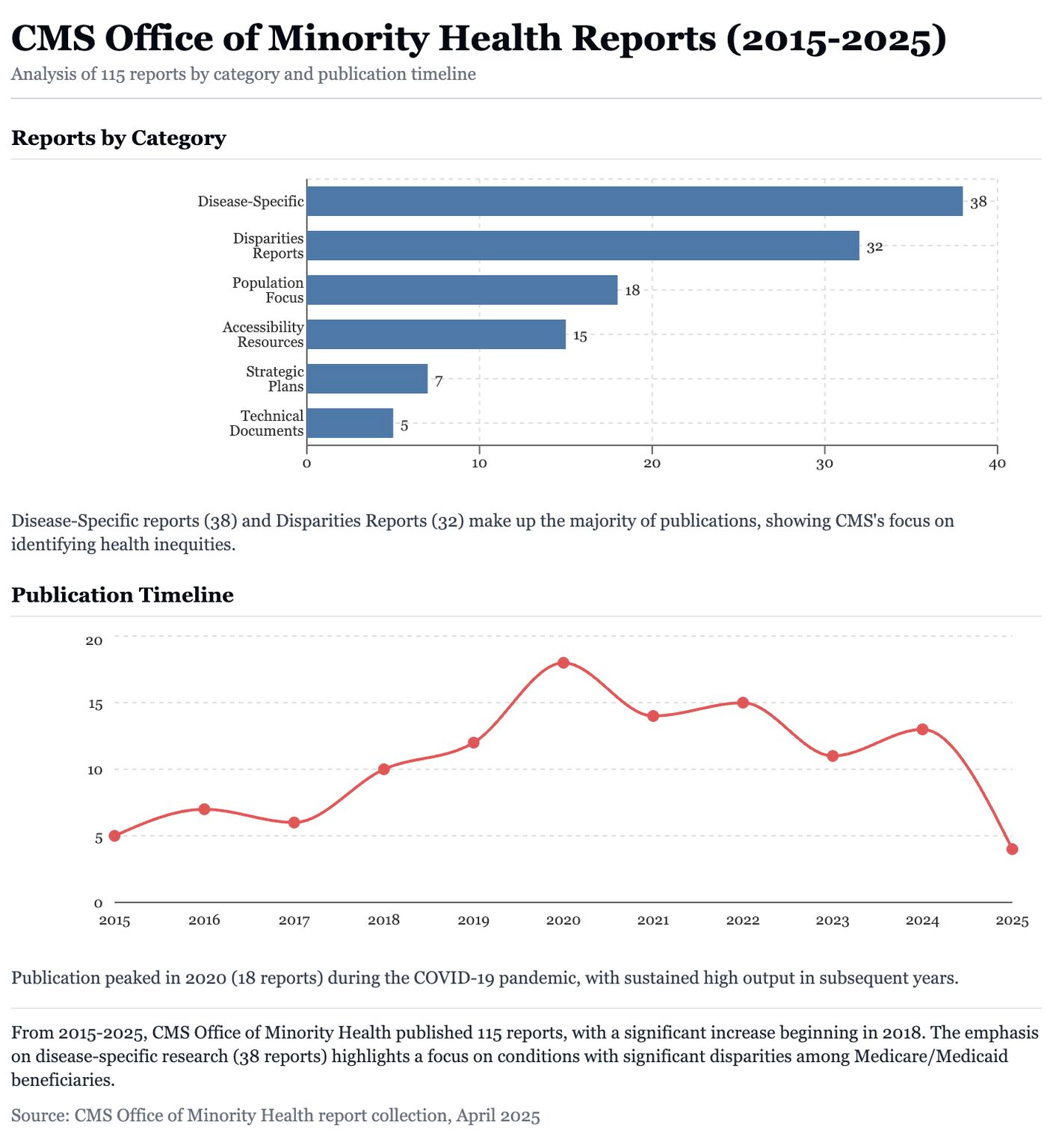
Yubin Park and Prince Baawuah archived the full database of CMS Office of Minority Health reports, including 100+ PDFs and Excel datasets following the recent layoffs. Their analysis using Claude.ai revealed decade-long trends in disease-specific research, population targeting, and accessibility strategies (see image below). The data is now preserved to ensure it’s not lost (LinkedIn, h/t Yubin Park)
Josh Dipzinski shared a behind-the-scenes look from Tennessee as Premier Home Dialysis Services prepares to certify its newest unit. Now in its fifth year, the company is expanding with three new units, nine SNF partnerships, and its first in-house hemodialysis den. “We’re redefining dialysis care by raising the standard for home and SNF patients nationwide” (LinkedIn, h/t Josh Dipzinski)
The University of Pittsburgh Medical Center (UPMC) shared a comprehensive blueprint for population health management (PHM) in CKD. Their approach includes EHR-enabled risk tracking, palliative care strategies, and PHM models for dialysis and transplant transitions—leveraging their integrated payer-provider system and diverse clinical network (Managed Healthcare Executive).
A new post by Rubicon Founders highlights risk stratification models as essential infrastructure for value-based care. In their latest Tech at the Crossing feature, they explored how AI-powered tools are shifting health systems from reactive to proactive, helping predict and manage patient risk before costly interventions are needed. (Rubicon Founders)
Jobs
APP Hospitalist (Greenville, NC) at Eastern Nephrology
Palliative APP (Birmingham, AL) at Monogram Health
Associate Director, Health IT at Novartis
Process Engineer at Vantive
Resource Specialist – CSW at Tufts Medical Center
Clinical Affairs Specialist (RN) at Diality
Sr Medical Science Liaison, Nephrology at Biogen
SVP, Clinical Dev. & Medical Affairs at Click Therapeutics
Transplant Manager at Eurofins
Associate Director – Health Outcomes Liaison – Renal at Eli Lilly
Rare Disease Account Manager at Calliditas
Manager, Life Sciences Partnerships at Flatiron Health
VP, Business Development (Industry Partnerships) at Breakthrough T1D
Head of Brand, Marketing, and Communications at Monogram Health
VP, Financial Planning & Analysis at Strive Health
…Find 20+ more roles at jobs.signalsfs.com
H.R.2544 - Securing the U.S. Organ Procurement and Transplantation Network Act: This bill modifies how the Health Resources and Services Administration (HRSA) funds and manages the Organ Procurement and Transplantation Network. This network is a public-private partnership that links the professionals involved in the U.S. donation and transplantation system. Historically, only one organization has received a contract for managing the network. The bill expressly authorizes HRSA to award multiple grants, contracts, or cooperative agreements to support the operation of the network and eliminates a cap on the amount of funding available for supporting the network. The bill also specifies that the network shall be operated through awards that are distinct from awards for supporting the operation of the network's board of directors. Additionally, the Government Accountability Office must review the historical financing of the network, including the use of registration fees.
For a more thoughtful and thorough discussion of all things #transplantation, please consider joining us in Slack to hear lived experiences, earned wisdom, and candid questions from our kidney forum.
![Signals From [Space]](https://substackcdn.com/image/fetch/$s_!IXc-!,w_40,h_40,c_fill,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F9f7142a0-6602-495d-ab65-0e4c98cc67d4_450x450.png)
![Signals From [Space]](https://substackcdn.com/image/fetch/$s_!lBsj!,e_trim:10:white/e_trim:10:transparent/h_48,c_limit,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F0e0f61bc-e3f5-4f03-9c6e-5ca5da1fa095_1848x352.png)