June brought a mix of momentum and reality checks in kidney care. On one hand, we’re living through a golden era of CKD therapeutics—new trials, combination therapies, and next-gen drug classes are redefining what’s possible. But grounded realities persist: screening rates remain low, transplant access remains unequal, and nearly $1 trillion in proposed Medicaid cuts for almost 300,000 dialysis patients raise dire concerns about erasing decades of progress and leaving people without options. Health care leaders and policymakers are weighing in on Medigap reforms and FDA actions to expand access, while the community rallies behind bold ideas, smart tech, and public-private innovation. As always, we’re here to surface and synthesize what this means for kidney health. Thanks for being here with us.1
Have tips or feedback? Hit reply or drop us a note here.
Reading time: 25 minutes
What’s Inside
News: FDA lifts REMS barriers for CAR-T access, China's GLP-1 landscape heats up, Delaware passes Medigap birthday rule, new CMS policies on drug price negotiations, Medicaid cuts stir concern, NKF-Sanford win UNIVANTS Award for CKD care, XS Innovations raises €1.8M for smart AVF valve…
Research: Smartphone CKD screening boosts diagnosis, KDIGO urges prevention-first kidney health strategies, machine learning predicts sAKI in sepsis, transplant fellowship report from AST, disparities in AKI recovery for dual-eligibles, high-volume transplant centers improve access, updated proficiency testing in immunogenetics, evolving views on patient experience measurement…
Community: PKD Foundation invests $2M in 2025 research grants, Edouard Fu highlights cystatin C findings at KDIGO symposium, NKF and Sanford Health win UNIVANTS award, voices call out Medicare Advantage care delays…
Events: Kasr Al Ainy Kidney Congress, AI Summit at Cleveland Clinic, Columbia Renal Biopsy Course, World Transplant Congress in SF, Budapest Nephrology School, SLANH Congress in Ecuador…
Jobs: Natera, ProKidney Corp., Akebia Therapeutics, Vantive, NVIDIA, Edwards, Gates Foundation, MannKind, Pair Team, Cityblock Health, Ardelyx…
This edition of Signals is brought to you by Natera. With over 130,000 patients tested with the Renasight™ test, Natera has the largest commercially available kidney disease database that can help accelerate research and drug development in the space. Discover how Natera’s Renasight™ database helped accelerate an Alport Syndrome clinical trial by 3x. Thanks team!
Visual of the Month

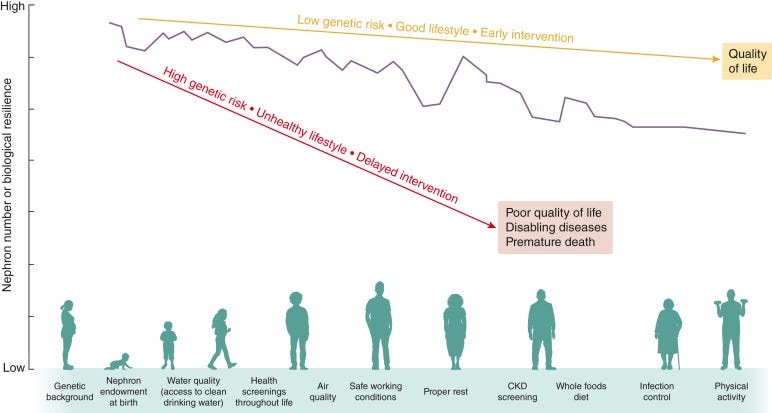
Charting the Golden Age of CKD Therapies. This month’s visual traces the remarkable evolution of chronic kidney disease (CKD) therapeutics—from single-agent ACE inhibitors in the early 1990s to today’s era of multidrug regimens. New classes like SGLT2 inhibitors, non-steroidal MRAs, GLP-1 receptor agonists, and endothelin receptor antagonists are no longer siloed—they’re converging in trial designs, guideline updates, and emerging standards of care. Once aspirational, combination therapy is now data-backed and poised to transform how we treat CKD.
But while we're living through a therapeutic renaissance, the sobering truth remains: you can’t treat what hasn’t been diagnosed. CKD screening—particularly in high-risk populations like those with diabetes—remains inconsistent in the U.S. and globally. As one observer put it, “We need to take a different approach to updating clinician behavior.” Breakthroughs in treatment must be matched by breakthroughs in detection and awareness if we hope to close the gap between innovation and impact.
News
POLICY
Senate bill would slash Medicaid by $1 trillion, KFF warns. A new KFF analysis projects that the Senate reconciliation bill would cut federal Medicaid spending by 15% over the next decade, with major impacts from work requirements, provider tax restrictions, and tighter enrollment rules. The five biggest provisions account for $896 billion—nearly 90% of the total—and would disproportionately affect expansion states. Louisiana and Virginia face the steepest losses, with projected cuts of 21% over the period (KFF).
NKF slams Senate Medicaid cuts as “devastating” for kidney patients. Following Senate passage of the “One Big Beautiful Bill,” the National Kidney Foundation warned that new work requirements, dialysis copays, and rural clinic funding cuts could disrupt care for nearly 300,000 dialysis patients on Medicaid. SVP Jesse Roach, MD, called the bill “a giant leap backward,” urging lawmakers to prioritize sustainable, equitable kidney care (NKF, h/t Jesse Roach, MD).
New ICD-10 codes recognize APOL1-mediated kidney disease. A major win for health equity and patients with AMKD, the new codes (N07.B and Z84.11) support earlier diagnosis, family history tracking, and targeted care for APOL1-related kidney disease—disproportionately affecting those of African ancestry (KHI, LinkedIn, h/t Ogo Egbuna, MD).
CMS unveils WISeR model as prior auth reform gains steam. The new WISeR model (Wasteful and Inappropriate Service Reduction) will test AI-assisted claim reviews in Medicare, rewarding vendors for denying unnecessary services. Days later, HHS announced a sweeping industry pledge from 50+ insurers—including SCAN, Aetna, and UnitedHealthcare—to streamline prior authorization with faster decisions, fewer delays, and more transparency. SCAN CEO Dr. Sachin Jain backed the effort publicly but warned that true reform will depend on listening to physicians and rebuilding trust with patients (AHIP, Forbes).
Drugmakers win $5B rollback in Medicare price negotiations. According to NYT, the new GOP-led bill expands exemptions for rare-disease drugs, weakening Medicare’s ability to negotiate prices and erasing nearly $5 billion in projected savings. Supporters say the change encourages companies to pursue additional rare disease indications, but critics warn it delays affordability while preserving pharma’s pricing power for blockbuster therapies (New York Times, h/t Dr. Benjamin Rome).
Turmoil deepens across U.S. science agencies. Hundreds of NIH employees signed the “Bethesda Declaration” protesting funding cuts, canceled research, and leadership decisions under Director Jay Bhattacharya. Just days later, NSF staff were blindsided by news that their Virginia headquarters would be handed over to HUD, displacing more than 1,800 employees without a clear relocation plan. The dual upheavals underscore growing concerns about political interference and long-term erosion of the U.S. scientific enterprise (STAT, Eos).
INDUSTRY & REGULATORY
China launches first homegrown GLP-1 therapy. Once known for producing cheaper versions of GLP-1 drugs like Ozempic, Chinese companies are now leading the next wave of metabolic treatments. Sciwind’s ecnoglutide showed up to 13.8 kg of weight loss in a phase III trial, while Innovent Biologics is advancing mazdutide—developed in partnership with Eli Lilly—which mimics GLP-1 and glucagon. With dozens of candidates in the pipeline, China is positioning itself as a serious contender in the global fight against obesity and diabetes (Nature, NEJM, h/t Manthan Mehta).
FDA approves first single-pill triple therapy for hypertension. GMRx2 combines telmisartan, amlodipine, and indapamide into one tablet, offering superior blood pressure reduction compared to dual therapies in clinical trials. The once-daily regimen is designed to improve adherence and simplify treatment for patients starting hypertension care (Pharm Times, h/t Pranav Garimella, MD)
DK Medtech and Mozarc partner to advance dialysis vascular access. The two companies announced a U.S. collaboration to distribute DK Medtech’s high-performance balloon catheters—DKutting™ and DKaptain™—designed to treat complex AV fistula lesions in dialysis patients. Mozarc will serve as exclusive U.S. distributor, aiming to improve access maintenance for over 500,000 patients (Press release).
Medtronic launches U.S. arm of renal denervation registry. The first U.S. patient has been treated in the GSR-DEFINE study, a global real-world registry evaluating the Symplicity Spyral RDN system for uncontrolled hypertension. With 5,000 patients planned across 55 countries, the trial aims to clarify the role of RDN in everyday U.S. practice and builds on global data showing sustained blood pressure reductions over three years (MassDevice).
Current Health cofounder buys company back from Best Buy. Christopher McGhee, who sold Current Health for $400M in 2021, has reacquired the healthcare-at-home platform and announced a “return to scrappiness” and startup focus. The move signals a renewed push to build independent, patient-centered solutions in the fast-growing home care market (MobiHealthNews, LinkedIn).
Parallel Bio raises $21M to scale human-first drug discovery. Backed by AIX Ventures and Marc Benioff, Parallel Bio’s platform uses immune organoids and AI to model patient biology and predict drug efficacy earlier in development. With 8 pharma partners and over 50 candidates in testing—including Centivax’s universal flu vaccine—the company aims to reduce R&D costs and failure rates by shifting discovery from animals to humans from day one (Press release).
Research
GENERAL
KDIGO calls for shift toward CKD prevention across the lifespan. A new consensus report reframes chronic kidney disease as part of a broader cardiovascular-kidney-metabolic (CKM) syndrome and urges earlier, personalized interventions—including lifestyle changes, risk prediction tools, and preventive use of SGLT2i and GLP-1 therapies. The update emphasizes moving beyond progression management to proactive kidney health maintenance (Kidney International, h/t Edgar Lerma, MD).
Smartphone-enabled testing boosts CKD detection in primary care. A pilot study of 4,000 Geisinger patients using Healthy.io’s Minuteful Kidney™ home uACR test showed a 2.5x increase in testing completion and higher rates of CKD diagnoses, nephrology referrals, and new ACEi/SGLT2i prescriptions compared to usual care. The study highlights how digital tools can close screening gaps and support earlier intervention in high-risk patients (ADA 2025, Healthy.io, Geisinger, Boehringer Ingelheim, h/t Alex Chang).
Dual-eligible dialysis patients less likely to recover kidney function after AKI. A retrospective study of over 2,500 patients found that those with both Medicare and Medicaid had significantly lower odds of regaining kidney function within six months, compared to those with Medicare, MA, or VA coverage. The findings highlight how social risk factors—before and after dialysis initiation—can influence recovery outcomes in AKI care (JASN, h/t Paul Gordon)
Dr. Edouard Fu spotlights cystatin C's value in GFR estimation. At the New Kids on the Block symposium, Dr. Fu shared key findings from his research showing that combined creatinine-cystatin C equations (eGFRcr-cys) outperform single-marker estimates and better predict outcomes—especially in older adults. His work supports KDIGO’s 2024 call to expand cystatin C use in clinical practice (LinkedIn, h/t Edouard Fu).
Concerns about Medicare Advantage marketing in dialysis. A large qualitative analysis found that aggressive, sometimes misleading advertising by MA plans and dialysis organizations may steer ESKD patients into plans with limited provider networks and higher profit margins for large dialysis chains. While some patients may benefit, the study highlights financial conflicts and calls for stronger guardrails to protect patient choice and outcomes (JAMA, h/t Eugene Lin, MD).
TRANSPLANT
Class II molecular mismatches linked to antibody-mediated rejection. A new study from teams in Paris, UPMC, and PIRCHE found that both HLA eplet mismatches and PIRCHE-II scores were independently associated with AMR in kidney transplant recipients, while HLA evolutionary divergence showed no correlation—limiting its value for population-level immune risk stratification (Nature, h/t Massimo Mangiola).
High-volume transplant centers offer better access and faster transplants. A national study of 196 U.S. kidney programs found that patients at high-volume centers were more likely to receive transplants, waited less time, and benefited from broader acceptance of marginal organs. In contrast, low-volume centers had lower acceptance rates and longer hospital stays—prompting calls for structural changes to improve equity and efficiency (Annals of Surgery).
The Lancet highlights global advances and disparities in solid organ transplantation. A new three-part series outlines breakthroughs in organ preservation, xenotransplantation, and precision immunosuppression, while also calling for bold policy reforms to address global inequities in transplant access and outcomes (The Lancet, h/t Alexandre Loupy, MD).
AST outlines strategies to strengthen transplant training pipeline. In response to declining interest in transplant fellowships, the American Society of Transplantation’s Fellows Task Force released recommendations focused on early exposure, education, flexible training paths, and improved work–life balance—aiming to attract and retain the next generation of transplant specialists (AJT, Ishna Poojary-Hohman, MD).
Global leaders call for modernization of immunogenetics proficiency testing. A new editorial in Frontiers in Genetics reviews lessons from 14 international programs, urging the H&I field to adapt PT schemes to meet the demands of emerging technologies, novel alleles, and clinical decision-making. Contributors from Europe and North America call for digital innovation, flexibility, and expanded testing to ensure quality and consistency in transplant diagnostics (Frontiers in Genetics, h/t Kelley Hitchman, PhD).
AI & MACHINE LEARNING
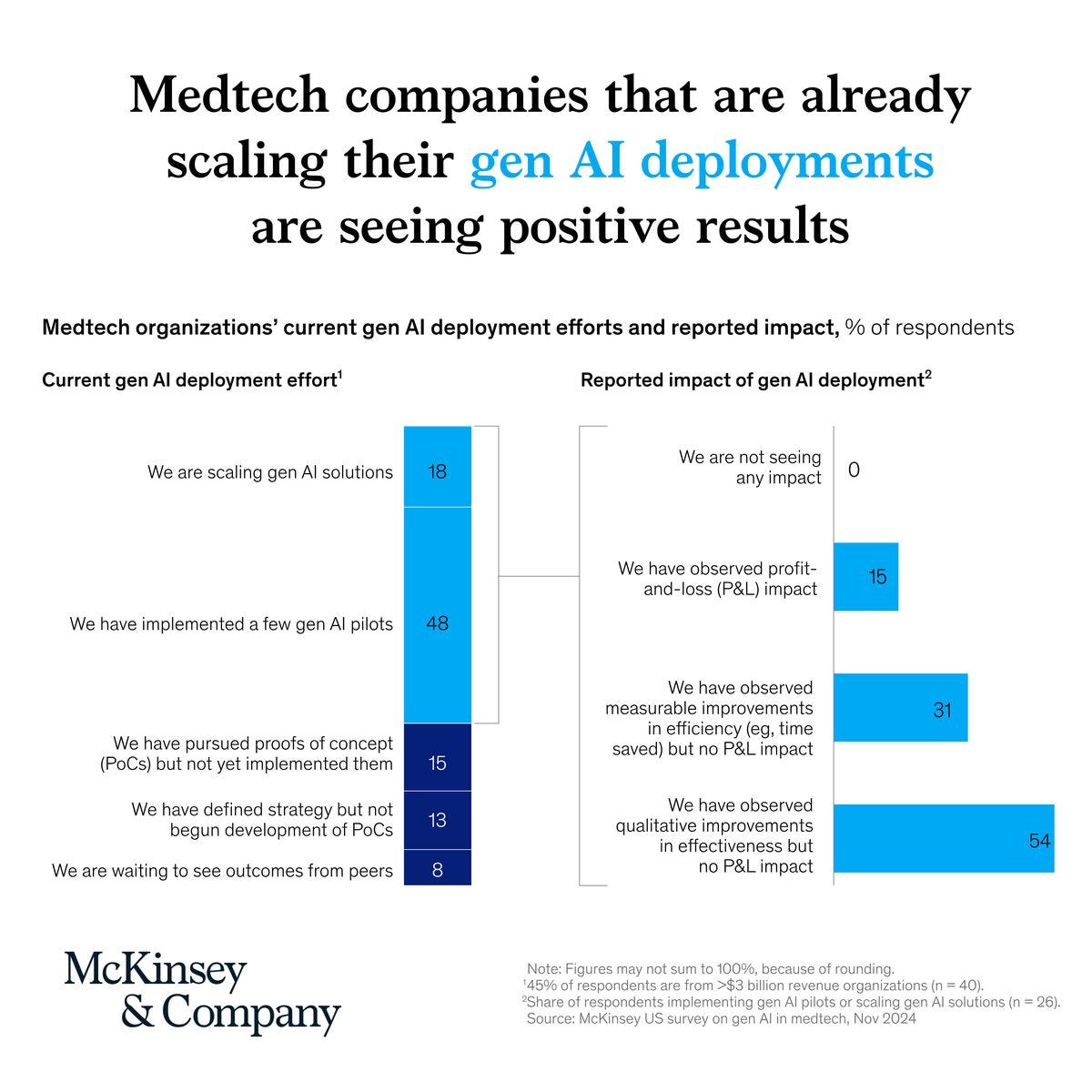
Gen AI could unlock $50B in new medtech revenue. McKinsey projects that medtech companies could generate over $50 billion annually from AI-powered product and service innovation, alongside $14–55 billion in productivity gains. Early adopters are using gen AI to accelerate R&D, automate compliance, and improve decision-making—with 15% already seeing a positive impact on their P&L (McKinsey).
Machine learning outperforms SOFA in predicting AKI in sepsis-related heart injury. In a study of 1,467 ICU patients with sepsis-induced myocardial injury, a random forest model predicted severe AKI within 7 days with an AUC of 0.81—significantly outperforming the traditional SOFA score (AUC 0.66). The findings highlight the potential of EMR-based models to guide early intervention in high-risk patients (Nature, h/t Rolando Claure Del Granado, MD).
JAMA calls for a new era in patient experience measurement. A Viewpoint article argues for modernizing tools like CAHPS, expanding access to feedback infrastructure, and integrating qualitative methods to restore trust and drive meaningful improvement. The authors urge health systems and policymakers to treat patient experience as core to quality—not a compliance exercise (JAMA, h/t Justin Schrager, MD).
Community
POLICY & REGULATORY
Senator Frist warns against Medicaid cuts. In a U.S. News op-ed, he calls the Senate’s proposed $1 trillion reduction a threat to rural health infrastructure, saying the sacrifices the legislation imposes on struggling communities don't improve the financial health of our nation (US News & World Report).
Delaware joins growing list of states advancing Medigap birthday rule protections. Legislation passed in June would allow Medigap enrollees a 60-day window following their birthday to switch to a plan with equal or lesser benefits without medical underwriting. If signed into law, Delaware would become the 13th state to adopt a “birthday rule,” part of a growing state-led push to expand guaranteed issue rights for Medigap enrollees amid calls to close federal gaps in coverage flexibility (MedicareResources.org, h/t Dialysis Patient Citizens).
Medicare Advantage under fire for deadly delays in cancer care. A powerful op-ed in The Hill tells the story of a patient whose cancer progressed amid months of prior authorization delays under Medicare Advantage. The authors argue that while the program is marketed as efficient and affordable, it too often fails seriously ill seniors—prompting calls for bipartisan reform to curb denials, cap out-of-pocket costs, and protect access to timely care (The Hill).
SRTR adds program-level trends to transplant data explorer. The updated Donation and Transplant System Explorer now includes weekly trend data at the transplant program and Organ Procurement Organization (OPO) level—offering long-requested visibility into local patterns using a 365-day lookback. SRTR hopes the new feature help programs better understand their own performance and drive improvements in care and outcomes. (SRTR, h/t Nicholas Wood).
FDA eyes home-based care models. A recent FDA Direct interview asks the CDRH Director “What if healthcare could happen at home - virtually?” The group talks through new initiatives including sensors and VR environments to shift care from hospitals to homes (FDA). Note: We also wrote a bit about the genesis of the “home as a health hub” program here.
FDA removes REMS barriers for CAR-T therapies to expand access. The agency removed Risk Evaluation and Mitigation Strategies (REMS) requirements for all currently approved BCMA- and CD19-directed CAR-T cell therapies, including Abecma, Breyanzi, Carvykti, Yescarta, and Kymriah. The move shortens post-infusion monitoring guidelines and lifts facility certification requirements, helping improve access—especially for rural patients—and allowing community cancer centers to administer these therapies (Fierce, h/t Vera Mucaj).
GENERAL
Renasolve expands rural dialysis access. Dakota Regional’s new inpatient dialysis program brings care closer to home, with support from Renasolve and trained local nursing staff (LinkedIn, h/t Jimmy Thomas)
PKD Foundation commits $2M to advance research in 2025. CEO Susan Bushnell announced new funding focused on drug repurposing, mental health, “mini kidney” models, and PLD—reflecting the Foundation’s continued investment in innovative science and early-career researchers (PKD Foundation, h/t Susan Bushnell).
XS Innovations builds smart AVF valve. Co-founded by Prof. Joris Rotmans and Toon Stilma, the LUMC spinout is developing a magnetically controlled implantable valve to open and close AV fistulas, aiming to reduce cardiovascular strain and vessel damage for dialysis patients. Backed by €1.8M in funding and a TTT voucher from RegMed XB/DCVA, the team is preparing for a first-in-human study by late 2026, with preclinical data expected this summer (RegMed XB).
NKF and Sanford win UNIVANTS award. In just 10 months, the partnership boosted CKD testing rates from 38% to 70%, CKD diagnoses from 20% to 73%, and SGLT2i prescriptions from <2% to nearly 10% across Sanford’s diabetic population, showcasing the power of embedding NKF’s CKD Change Package into rural health systems (UNIVANTS, h/t Elizabeth Montgomery).
Organ preservation tech gains traction. From CORE’s adoption of the OrganOx system for liver viability to Inova’s first transplant using the Paragonix KidneyVault, a wave of next-gen preservation tools is starting to reshape how organs are evaluated, transported, and transplanted.
Events Calendar
Kasr Al Ainy Annual Kidney Congress (July 10-11 in Cairo, Egypt)
AI Summit for Healthcare Professionals (July 11 at Cleveland Clinic)
Columbia Renal Biopsy Course 2025 (July 16 in New York, NY)
World Transplant Congress (August 2-6 in San Francisco, CA)
29th Budapest Nephrology School (August 25-29 in Budapest)
SLANH 2025 Congress (August 27-30 in Guayaquil, Ecuador)
Jobs
Bilingual Nephrologist — Miami, FL (Contact us)
Sr Dir, Product Mgmt – Transplant — Natera
Director, Process Development — ProKidney Corp.
Medical Director, Clinical Research — Akebia Therapeutics
Assoc Director of R&D – Acute Systems — Vantive
Senior Director, Engineering R&D — Edwards
Deputy Director, Emerging Innovations — Gates Foundation
VP, Sales & Marketing — MannKind
Associate Medical Director (VBC) — Pair Team
Senior Data Analyst, Clinic Ops — Cityblock Health
Director, Field Medical Affairs — Ardelyx
Browse 100+ more at jobs.signalsfs.com…
Work with Signals Group
Want to help shape what comes next in kidney health? Signals Group is expanding and finding new ways to support the growth of this community. Whether you’re looking for new talent, visibility, or expertise, we’d love to hear from you.
Share — Tell us what unmet needs you’d like to see solved
Sponsor — Share your work with 15,000 kidney professionals
Subscribe — Support independent kidney news and research
Grow — Partner with us to reach your next company milestones
This audio summary may include variations in pronunciation and is intended for informational purposes only. For complete accuracy and source attribution, please refer directly to the original written materials and cited sources. Always consult trusted references when interpreting medical or scientific content.
![Signals From [Space]](https://substackcdn.com/image/fetch/$s_!IXc-!,w_40,h_40,c_fill,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F9f7142a0-6602-495d-ab65-0e4c98cc67d4_450x450.png)
![Signals From [Space]](https://substackcdn.com/image/fetch/$s_!lBsj!,e_trim:10:white/e_trim:10:transparent/h_48,c_limit,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F0e0f61bc-e3f5-4f03-9c6e-5ca5da1fa095_1848x352.png)
![Signals From [Space]](https://substackcdn.com/image/fetch/$s_!NnOt!,w_144,h_144,c_fill,f_auto,q_auto:good,fl_progressive:steep,g_auto/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F688fc47b-7202-4a2e-b4f4-fea2b047ab1b_1500x1500.png)