TL;DR
A new survey of more than 10,000 solid organ transplant recipients offers the clearest picture yet of what life on lifelong immunosuppression really looks like, and the results are sobering. Nearly all respondents (92%) reported side effects, with more than half saying those effects have a “moderate” or “great deal” of impact on their daily lives. One in four skip doses because of side effects; nearly 40% skip fills or refills because of cost. Yet in the last 15 years, the immuno drug toolbox has barely changed. This gap between need and innovation is one of transplant’s biggest under-told stories— and now we have the data to show it.
What Happened
The American Society of Transplantation and a task force of transplant leaders launched a survey to capture transplant recipients’ own experiences with immunosuppressive therapy. Over 16 months, they gathered more than 14,000 responses, validated 10,091 of them, and built what is now the largest dataset of its kind. In fact, this is the largest and most comprehensive survey of organ transplant recipients’ perceptions of immunosuppression use and treatment experiences ever undertaken.1
The survey includes diverse representation from 232 transplant centers across the U.S. and Canada. The median time since transplant was just shy of 7 years, meaning these are voices of long-term survivors: people who’ve navigated rejection risk, side effects, follow-up visits, and the slow grind of chronic conditions brought on or worsened by the very drugs keeping their organs alive. This is what they told us.
Key findings
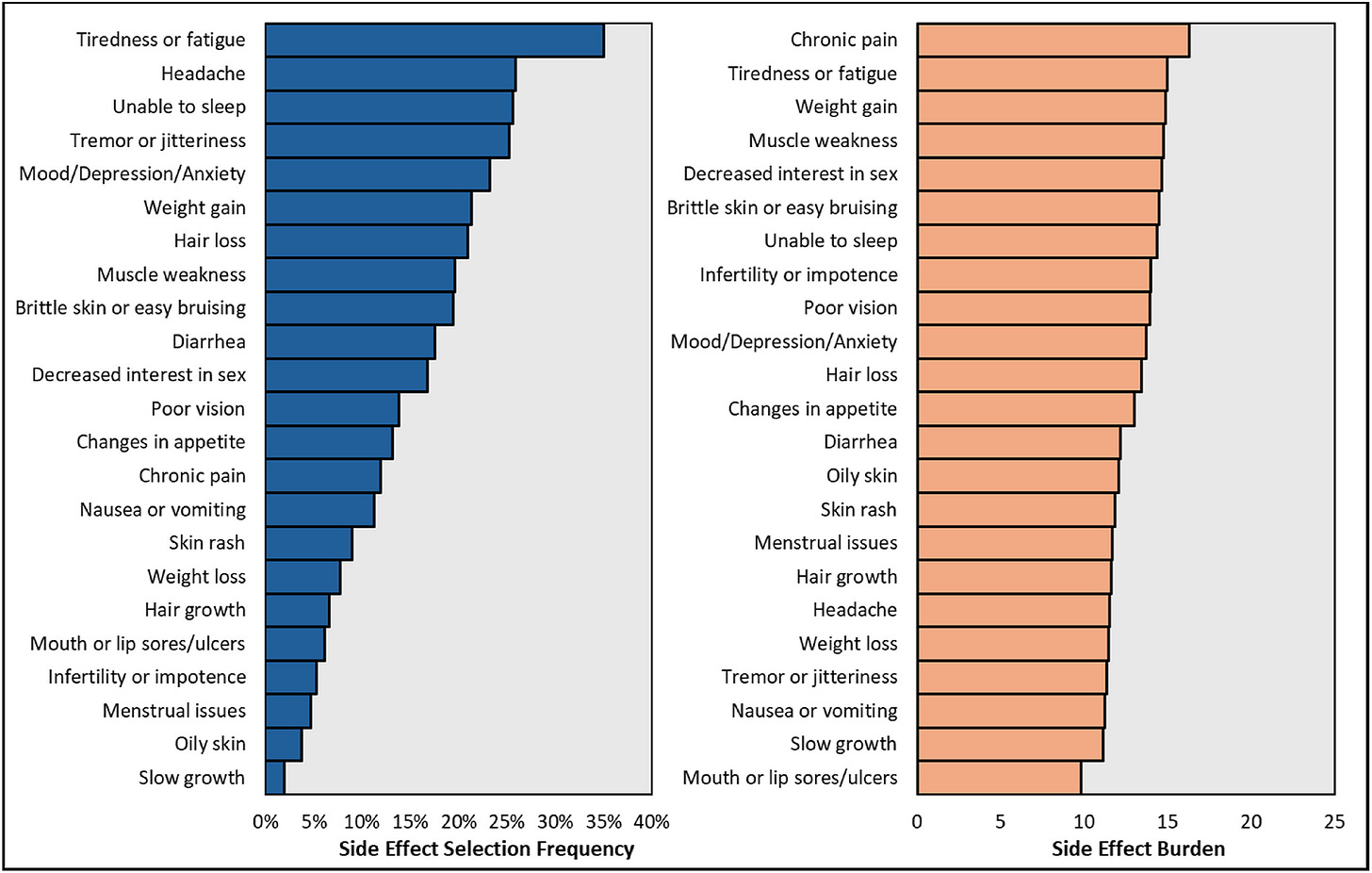
Side effects are near universal: 92% reported at least one; median of three. Most occurred “often” or “always.”
Daily impact is high: 54% rated the effect on their lives as moderate or worse. Top burdens included fatigue, pain, skin cancer, brain fog, and diabetes.
Adherence suffers: 25% skipped doses due to side effects; 40% skipped refills due to cost.
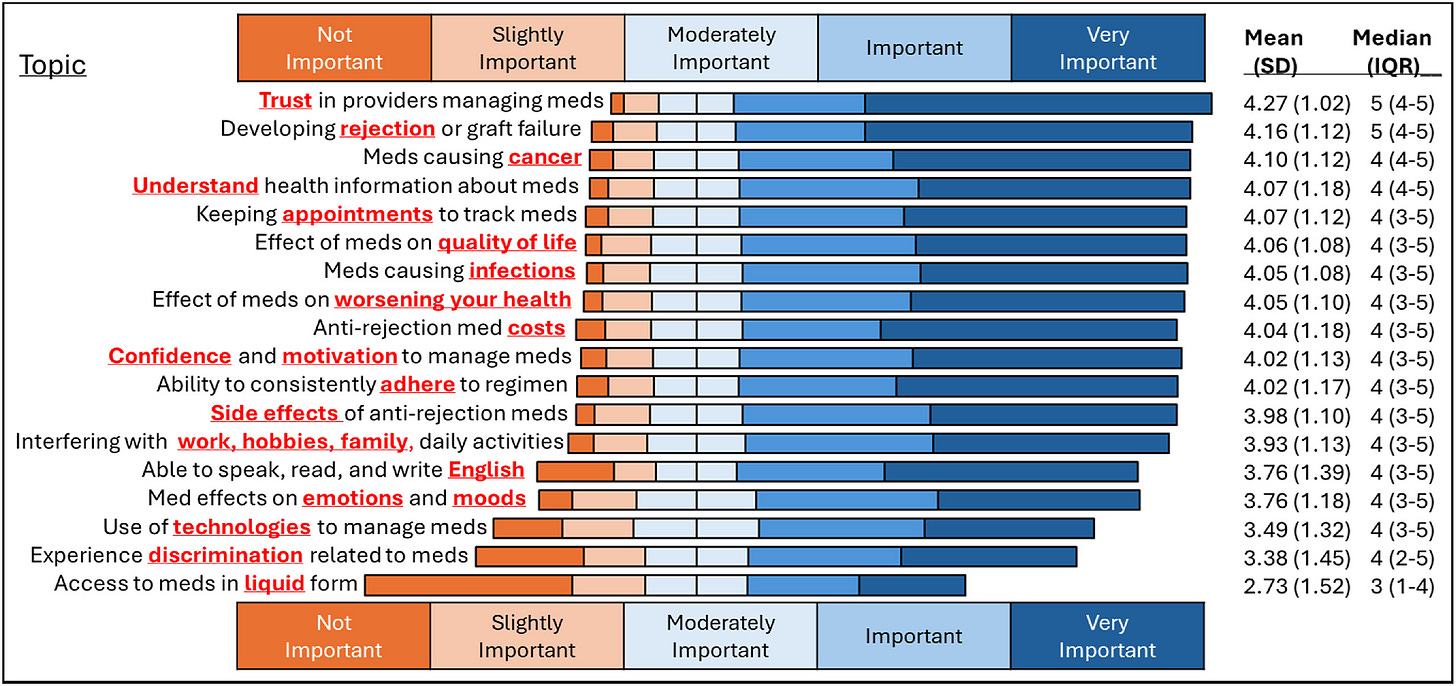
Trust is high: Most reported strong trust in their care teams and confidence in their own medication management, but still faced barriers that trust alone can’t solve.
For patients, these aren’t abstract numbers. One kidney recipient put it plainly:
Prednisone caused severe mood swings, insomnia, and anxiety. Physically, it led to rapid weight gain, fragile skin, slow healing, muscle weakness, high blood pressure, and worsening bone pain. [Mycophenolate] brought relentless gastrointestinal problems—nausea, cramping, diarrhea, and stomach upset. These are horrible drugs.”
Why It Matters
Transplant medicine is one of the great success stories of modern healthcare, but the AST survey makes clear that success has come at a steep cost. For many recipients, survival means trading one set of risks for another: fatigue, skin cancers, diabetes, and the constant financial and psychological strain of lifelong therapy.
The survey’s findings strip away any doubt about what’s at stake:
Therapeutic inertia has a cost. The price isn’t just measured in graft survival, but in the daily toll on quality of life. Imagine waiting years for a kidney, enduring dialysis, and finally receiving a transplant—only to face a new set of daily burdens like those described above.
The innovation gap is structural. FDA and EMA endpoints reward short-term graft outcomes over long-term safety, tolerability, and patient-reported outcomes. Remember, we have basically “maximized” 1-year graft survival (more on that below).
Cost is still a barrier. Patients rank it at the top, alongside trust and rejection risk. Even after the immuno coverage bill took effect, financial strain still drives nonadherence. Before the benefit, patients paid $10,000–$14,000 a year for these drugs; with it, premiums are closer to $3,000 — still a steep price for many.2
This isn’t just about making better drugs; it’s about changing the rules so better drugs can actually be developed, approved, and used. When Congress passed the immuno coverage bill in 2020, the CBO estimated that preventing graft failures could save $400 million over the decade (dialysis is expensive!). The lesson is clear: we don’t just need better therapies, we also need better goalposts and safeguards.
Background
If you trace the history of transplant therapeutics, the story starts fast and then slows to a crawl. The 1990s and early 2000s saw a burst of innovation — tacrolimus, mycophenolate, sirolimus, even entirely new classes like co-stimulation blockers. But since belatacept’s approval in 2011, the pace has been almost glacial.3 Most of what’s come to market in the last decade has been a reformulation or an expanded label, not a fundamentally new way of protecting the graft.
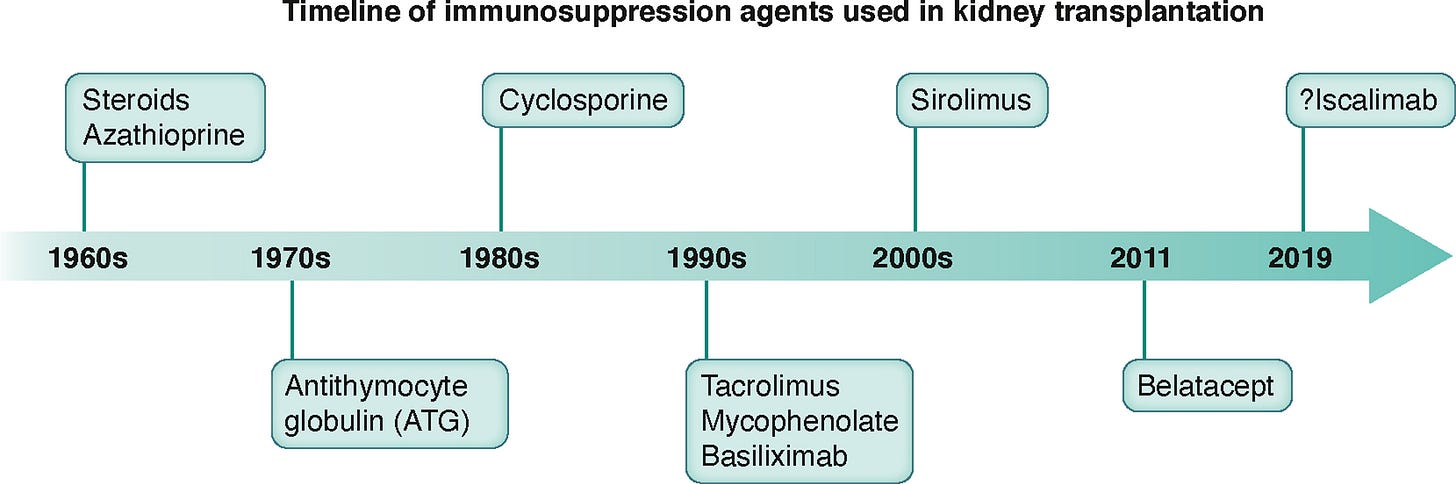
That long pause isn’t for lack of ideas. The real bottleneck has been the rules of the game: trial endpoints and regulatory pathways that reward short-term graft outcomes over long-term safety, tolerability, or quality of life. For patients living with the side effects outlined in the AST survey, it’s a classic mismatch between what’s measured and what’s felt.
We measured 1-year outcomes, and look what happened: we’ve nearly perfected them.
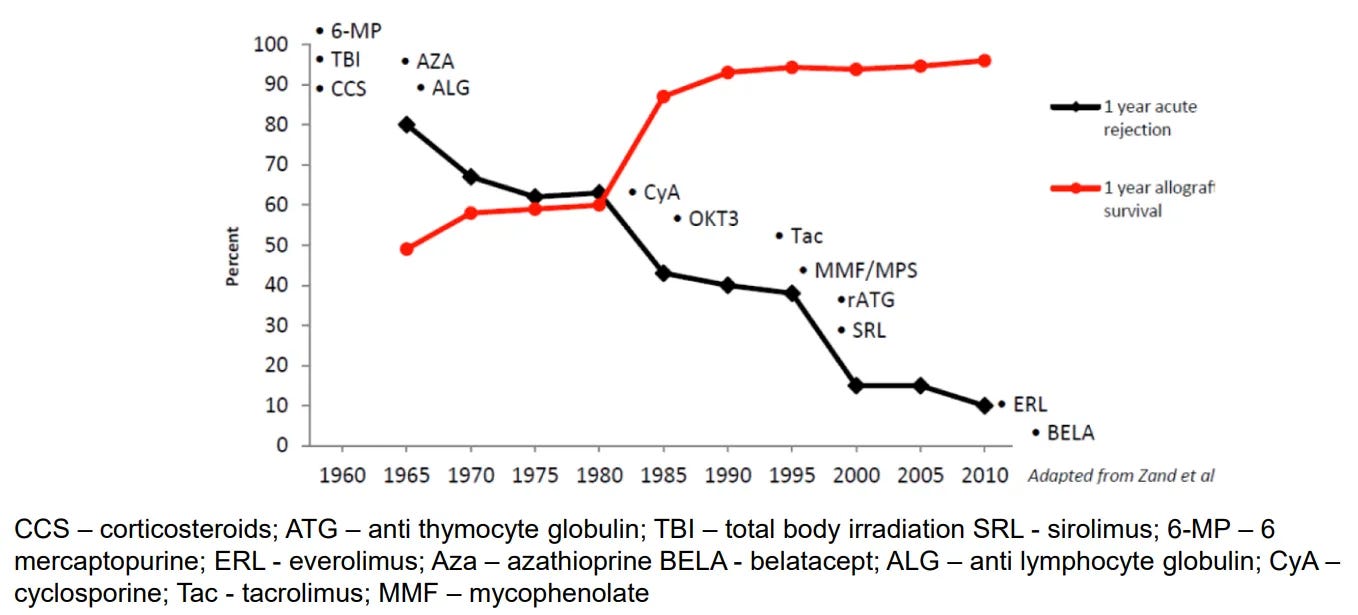
That’s why the iBox Scoring System has people’s attention. It’s a composite score (kidney function (eGFR), protein in the urine, donor-specific antibodies, sometimes paired with biopsy results) that predicts long-term graft survival. The EMA has already qualified it as a secondary endpoint. Now the FDA is reviewing it for full qualification as a “reasonably likely surrogate endpoint” under the accelerated approval pathway. In plain terms: if approved, it would let new transplant drugs be evaluated faster, with smaller trials, and still satisfy regulators that they’re making a meaningful difference.
And it’s not just iBox. The regulatory conversation is widening to include tools that could make transplant drug development more nimble: real-world evidence to supplement or replace some trial arms; digital health platforms to reduce patient burden and improve follow-up; and patient-centric trial designs that integrate quality of life metrics, adherence strategies, and economic considerations from the start.
It’s a promising mix to be sure, but only if the science, the funding, and the patient voice actually converge in the same place and at the right time.
What to Watch
The most immediate signal to watch is the FDA’s pending decision on the iBox Scoring System. Now in a 10-month review window, it could become the first transplant-specific surrogate endpoint qualified for accelerated approval — a change that would mean shorter, smaller, and more targeted clinical trials.
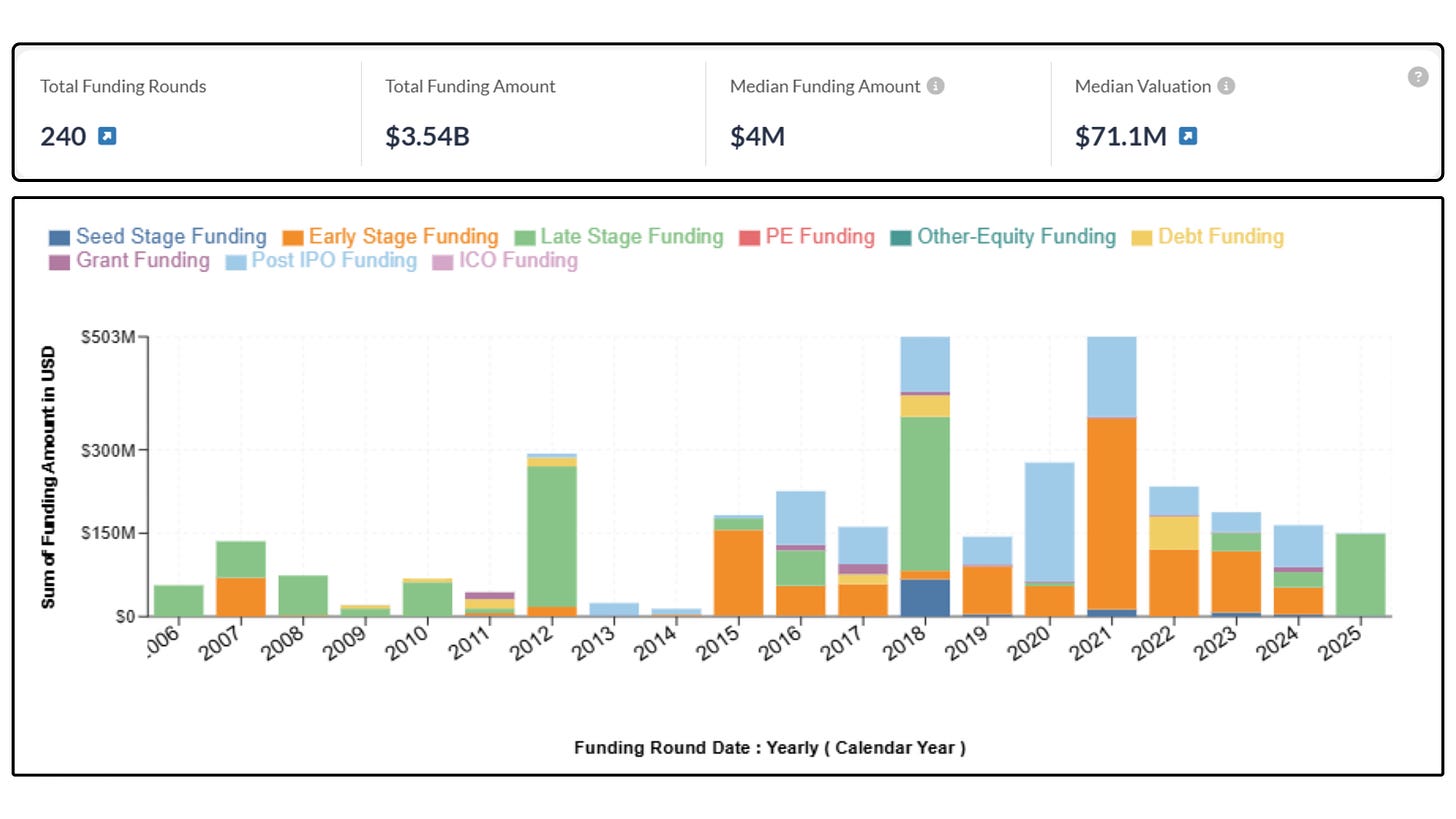
Money is already flowing in behind that potential. More than 90 companies have raised over $3.5 billion for transplant research in the past five years, from pharma heavyweights like AstraZeneca, Novartis, and Sanofi to emerging biotechs and smaller startups you’ve likely never heard of.4 Many of them are looking at ways to rethink the immunosuppressive playbook entirely, whether through new mechanisms, combination regimens, or adjunctive drugs that tackle side effects head-on.
And the way those drugs get tested is changing. Trial design is starting to borrow from rare disease and cell/gene therapy playbooks: using AI to refine patient selection, relying on biomarker-driven endpoints, and designing studies that are leaner but more focused. It’s an approach that could cut years off the development timeline, but only if it keeps patient-reported outcomes in the frame. The AST survey just gave the field a crystal-clear set of those priorities; the question is whether developers will act on them.
What’s Next
If iBox clears the FDA, the first real wave of new immunosuppressants and transplant-adjacent therapies in over a decade could finally break through. Some will tackle broad rejection risk; others may target toxicities like cancer, diabetes, or neurocognitive effects. Either way, the next era looks more precise, with regimens calibrated to patient risk rather than a one-size-fits-all approach. I can get on board with that version of the timeline.
But science alone won’t get us there. Regulators, payers, and advocates will need to agree on a more patient-centered definition of “success” — one that values living well with a transplant as much as keeping it. The AST survey gives that case real teeth, putting hard numbers behind the hidden costs of today’s status quo.
At the same time, the horizon of transplant medicine is widening beyond traditional immunosuppression. The field is experimenting with entirely new playbooks — from AI-guided dosing and smarter drug delivery systems to cell-based therapies, xeno-transplantation, and regenerative medicine. The next decade could look very different from the last.
Here’s a glimpse of how that might look:
![Signals From [Space]](https://substackcdn.com/image/fetch/$s_!IXc-!,w_40,h_40,c_fill,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F9f7142a0-6602-495d-ab65-0e4c98cc67d4_450x450.png)
![Signals From [Space]](https://substackcdn.com/image/fetch/$s_!lBsj!,e_trim:10:white/e_trim:10:transparent/h_48,c_limit,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F0e0f61bc-e3f5-4f03-9c6e-5ca5da1fa095_1848x352.png)
![Signals From [Space]](https://substackcdn.com/image/fetch/$s_!NnOt!,w_144,h_144,c_fill,f_auto,q_auto:good,fl_progressive:steep,g_auto/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F688fc47b-7202-4a2e-b4f4-fea2b047ab1b_1500x1500.png)