The past month was a reminder of how fast kidney care is evolving and how interconnected our community has become in driving that change. Between the RPA Advocacy and Innovation Weekend and the sprint ahead to Kidney Week in Houston, it is clear that the field is entering a defining stretch. Policy decisions big and small, AI breakthroughs, and biotech milestones are converging in ways that will shape how patients experience care for decades to come. New data in AJMC offers one of the clearest signals yet that value-based care can slow kidney disease progression, with significantly reduced eGFR decline in advanced CKD stages. For a treatment modality and care model that have changed little for generations, it now feels as if we are testing the limits of that inertia from several angles at once.
From new FDA clearances and phase 3 wins to research redefining endpoints and equity in care, this month’s recap traces the threads linking science, industry, and policy. We also saw voices across the community, from patient advocates to transplant pioneers, pushing for more transparency, data sharing, and patient-centered progress.
If you will be at Kidney Week in a few weeks, I would love to connect. Reach out so we can find time to meet in Houston.1
Have tips or feedback? Hit reply or drop us a note here.
Reading time: 40 minutes
What’s Inside
News: Roche + Klinrisk AI kidney risk tool, Renalytix–Tempus partnership, Vertex pipeline advances, Hansa phase 3 win, Simergent Archimedes clearance, Virta $160M milestone, Mayo stem cell therapy, Nobel Prize immune tolerance, ASN announces $6M bridge grants…
Research: VBC slows CKD progression, a new era in lupus treatment, CRISPR-GPT gene-editing system, PITOR xeno immune mapping, Hypertensive kidney deaths rise 98%, Depression lowers CKD quality of life, Nanomedicine translation roadmap, Nephrology-dentistry survey…
Community: Rare & Genetic Kidney Summit collaboration, CKM trial redesign framework, Agentic AI in life sciences, PARASOL expands to APOL1 and MN, GlomCon Hawaii global updates, Maui lung transplant story, Nick Mangold’s kidney search
Events: Countdown to a Cure NYC (Oct 22), ASN Kidney Week (Nov 3–6, Houston), MedTech Strategist (Nov 19–21, San Diego), VEITH Symposium (Nov 18–22, NYC), Int’l Transplantation Science (Nov 16–19, San Diego), APCH (Nov 27–29, Lucknow)
Jobs: Kaüna, Renvio, Novartis, Travere, Otsuka, Cityblock, NYU, Ardelyx, Calliditas, Akebia, Genentech, Thermo Fisher, Natera…
This issue is made possible by Natera. Natera supports personalized kidney care and drug development through advanced genetic testing and insights. RenasightIQ™ is a robust clinicogenomic database of 170,000+ chronic kidney disease patients enriched with longitudinal clinical and claims data. This powerful platform delivers real-world insights from early investigation to commercialization to accelerate the kidney disease drug development lifecycle.
News
INDUSTRY & MARKET MOVES
Klinrisk and Roche launch partnership to expand CKD prediction globally.
Roche, in collaboration with Klinrisk, has received the first CE-mark approval for its AI-based risk stratification tool designed to assess progressive kidney function decline. The Kidney Klinrisk Algorithm will debut as part of Roche’s new CKD algorithm panel, alongside the Kidney KFRE Algorithm, to help clinicians evaluate patient risk across all stages of disease—including early, asymptomatic phases. The launch marks a major step in bringing predictive, regulatory-grade AI tools into global kidney care. (Roche, h/t Navdeep Tangri)Renalytix and Tempus AI partner to expand access to precision kidney testing. The definitive agreement will broaden availability of the FDA-approved, Medicare-reimbursed KidneyIntelX.dkd prognostic test across Tempus’ U.S. network of healthcare institutions. The collaboration will integrate the test into clinical workflows for patients with type 2 diabetes and chronic kidney disease, an estimated 15 million Americans, enabling risk stratification and earlier interventions to slow disease progression. Executives from both companies highlighted the partnership’s potential to drive multi-modal data innovation across the cardio-renal-metabolic space. (Press release, H/t Elise Wilfinger)
Simergent earns FDA clearance for its Archimedes home dialysis system.
Simergent’s automated peritoneal dialysis (APD) cycler received 510(k) clearance following successful usability testing with patients and nurses at Indiana University and Northwestern. The device enables quiet, mobile, in-home treatments and is designed to expand access to home dialysis for people living with kidney failure. The company is planning a U.S. launch of Archimedes in 2026. (Press release, h/t James Sloand, MD)NKF Innovation Fund backs BMI OrganBank to advance organ preservation.
Through this investment, BMI OrganBank will accelerate development of next-generation technologies designed to reduce kidney discards and increase the number of viable transplants. The move underscores NKF’s growing role as a strategic investor, bridging the gap between discovery and delivery and helping innovators bring more options to patients faster. (NKF, h/t Kevin Longino)Virta Health announced it has exceeded $160 million in annualized revenue, growing more than 80% year-over-year as employers and health plans seek sustainable alternatives to GLP-1 medications. Serving over 12 million covered lives and 550 partners, Virta’s nutrition-first model delivers weight loss outcomes on par with GLP-1s while lowering costs and reducing long-term drug dependence. The company’s rapid growth positions it among digital health’s fastest-scaling platforms addressing the root causes of metabolic disease. WDYT, till we see an IPO in 2026? (Press release, h/t Matthew Holt)
Weave raises $20M Series A to expand AI-native regulatory platform. The funding will accelerate product development and global expansion of the company’s platform, which streamlines regulatory workflows across the entire therapeutic lifecycle—from new drug and biologics applications to post-market submissions. Designed for biopharma and CRO teams, Weave combines human expertise with AI-driven document authoring, review, and publishing to reduce time, effort, and cost in complex regulatory filings. (Press release, h/t Brandon Rice)
DRUGS, DISCOVERY, & SCIENCE
Biotech sentiment improves as IPOs and deal flow pick up. While still early, John Carroll notes modest strengthening across biotech metrics in Q3, with a recovering XBI index and a few successful IPOs signaling renewed investor confidence. Observers like John Carroll suggest the worst may be over, as deal-making and R&D optimism return to the sector. (Endpoints (paywall), h/t John Carroll)
Novartis meets Phase III endpoint in IgA nephropathy. In the final APPLAUSE-IgAN trial analysis, Fabhalta (iptacopan) slowed kidney function decline and significantly improved eGFR slope over two years versus placebo. Fabhalta received accelerated approval for reduction of proteinuria in adults with IgAN in US in 2024; data support 2026 submission for traditional FDA approval (Press release, h/t Pranav Garimella, MD)
Vertex reported key milestones across its kidney pipeline, including FDA Breakthrough Therapy Designation for povetacicept in IgA nephropathy and completed enrollment for the AMPLITUDE Phase 2/3 trial of inaxaplin in APOL1-mediated kidney disease. Both programs could advance to accelerated approval in 2026 pending positive interim results. The company also launched a Phase 2 study of VX-407 in polycystic kidney disease, reinforcing its momentum toward first-in-class therapies that address the underlying causes of serious kidney disorders. (Press release, h/t Ogo Egbuna)
Hansa Biopharma reported positive Phase 3 results for its kidney transplant therapy, bringing its antibody-cleaving enzyme imlifidase closer to U.S. approval. The late-stage study met its primary endpoint, showing statistically significant improvement in kidney function at 12 months among highly sensitized patients who received imlifidase prior to transplantation. Already conditionally approved in Europe and several other countries, the therapy rapidly inactivates IgG antibodies to enable successful donor matches for patients previously unable to receive transplants. Hansa plans to pursue an accelerated FDA review following the Phase 3 win. (Fierce Pharma, press, h/t Kevin Fowler)
Mayo Clinic researchers reported promising results using stem cells to improve dialysis access durability, showing that transplanting a patient’s own stem cells into the vein before arteriovenous fistula (AVF) surgery helped prevent inflammation and narrowing. In a Phase I trial, patients receiving the therapy experienced faster healing and more durable AVFs—potentially extending the time they can remain on dialysis before needing a transplant. The cells’ anti-inflammatory properties appear to drive the benefit, and researchers have identified genetic biomarkers that may predict who responds best, paving the way for more personalized treatments in end-stage kidney disease. (Mayo Clinic, h/t Paul Gordon)
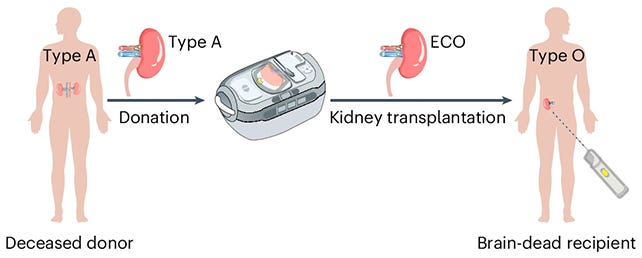
Scientists create ‘universal’ kidney compatible with any blood type. In a landmark collaboration between Canadian and Chinese researchers, scientists successfully converted a type A kidney into a universal type O organ using specialized enzymes. The organ functioned in a human model for several days, suggesting the potential to dramatically shorten transplant wait times in the future. (Nature, Science Alert, h/t John Barclay)
The 2025 Nobel Prize in Physiology or Medicine honored pioneers of immune tolerance. The assembly recognizing Mary Brunkow, Fred Ramsdell, and Shimon Sakaguchi for their discovery of regulatory T cells and peripheral immune tolerance. Their groundbreaking work redefined modern immunology and laid the foundation for advances still to come in transplantation and xeno-transplantation, helping researchers better understand how the body balances immune defense with long-term graft survival. (Nobel Prize, h/t Alex Loupy)
Dr. Graham Abra highlighted a new era of lupus nephritis therapy, noting rapid progress in treatment options after decades of limited innovation. Recent FDA approvals for belimumab and voclosporin (plus promising data for obinutuzumab) mark a shift toward combination regimens that minimize steroid exposure and improve kidney outcomes. With multiple late-stage trials now exploring agents like CAR-T, secukinumab, and ianalumab, guidelines from ACR, EULAR, and KDIGO increasingly endorse tailored, multi-mechanism approaches. The result is a more hopeful landscape for patients and clinicians alike, as lupus nephritis management moves toward safer, more personalized care. (Healio, h/t Graham Abra)
POLICY & REGULATORY
MedPAC canceled its October meeting as federal shutdown disruptions ripple through policy workstreams, delaying agenda items tied to Medicare payment reform and dialysis reimbursement updates. The pause could push back deliberations on long-term adjustments to ESRD policy and physician payment models. (MedPac, September transcript, h/t Mark Newsom)
CMS advanced two new drug pricing models for federal review, submitting the Global Benchmark for Efficient Drug Pricing (GLOBE) and Guarding U.S. Medicare Against Rising Drug Costs (GUARD) models to the Office of Management and Budget. Both are expected to test new approaches to benchmark pricing and mandatory participation for select entities, signaling a more assertive phase in Medicare’s drug cost containment strategy. (Advisory Board, Akin, h/t Jeffrey Davis)
Telehealth flexibilities for nephrology practices face uncertainty amid the government shutdown, raising concerns about a lapse in remote care coverage. While Congress broadly supports extending or making these flexibilities permanent, the timing of a new Medicare extenders package remains unclear—prompting clinics to adjust schedules and contingency plans until new guidance is issued. (CMA, h/t Robert Blaser)
CMS submitted the ESRD PPS and HOPPS rules for review at OMB, maintaining progress on regulations with statutory deadlines despite ongoing government gridlock. The submission marks a procedural milestone toward finalizing next year’s End-Stage Renal Disease Prospective Payment System and Hospital Outpatient Prospective Payment System updates. (LinkedIn, h/t Mark Newsom)
HHS reinstated health statistics staff involved in the National Health and Nutrition Examination Survey (NHANES) after initially issuing widespread layoff notices that alarmed public health experts. The reversal restores eight key planners responsible for coordinating national data collection on obesity, environmental exposures, and chronic disease—metrics central to federal nutrition and prevention policy. Officials said the mistaken terminations were caused by “data discrepancies and processing errors” during a larger round of 1,700+ layoff notices. The episode underscores continued tensions within HHS over balancing administrative restructuring with the need for reliable, long-term health data. (Axios, STAT)
ASN’s KidneyCure Foundation will commit $6 million in bridge grants to sustain kidney research, responding to recent NIH funding cuts that have jeopardized university labs and early-stage investigators. The 3-year initiative will provide one-year grants to researchers who would have received NIH R01 awards under previous paylines, helping preserve momentum in kidney science during a period of reduced federal support. “Fewer researchers will receive NIH funding in any fiscal year and the rate of new discoveries with clinical implications will slow down,” said Deidra Crews, chair of the KidneyCure board. The foundation hopes the program will protect critical talent and sustain discovery until NIH funding levels recover. (Healio, h/t Deidra Crews and Zach Cahill)
Research
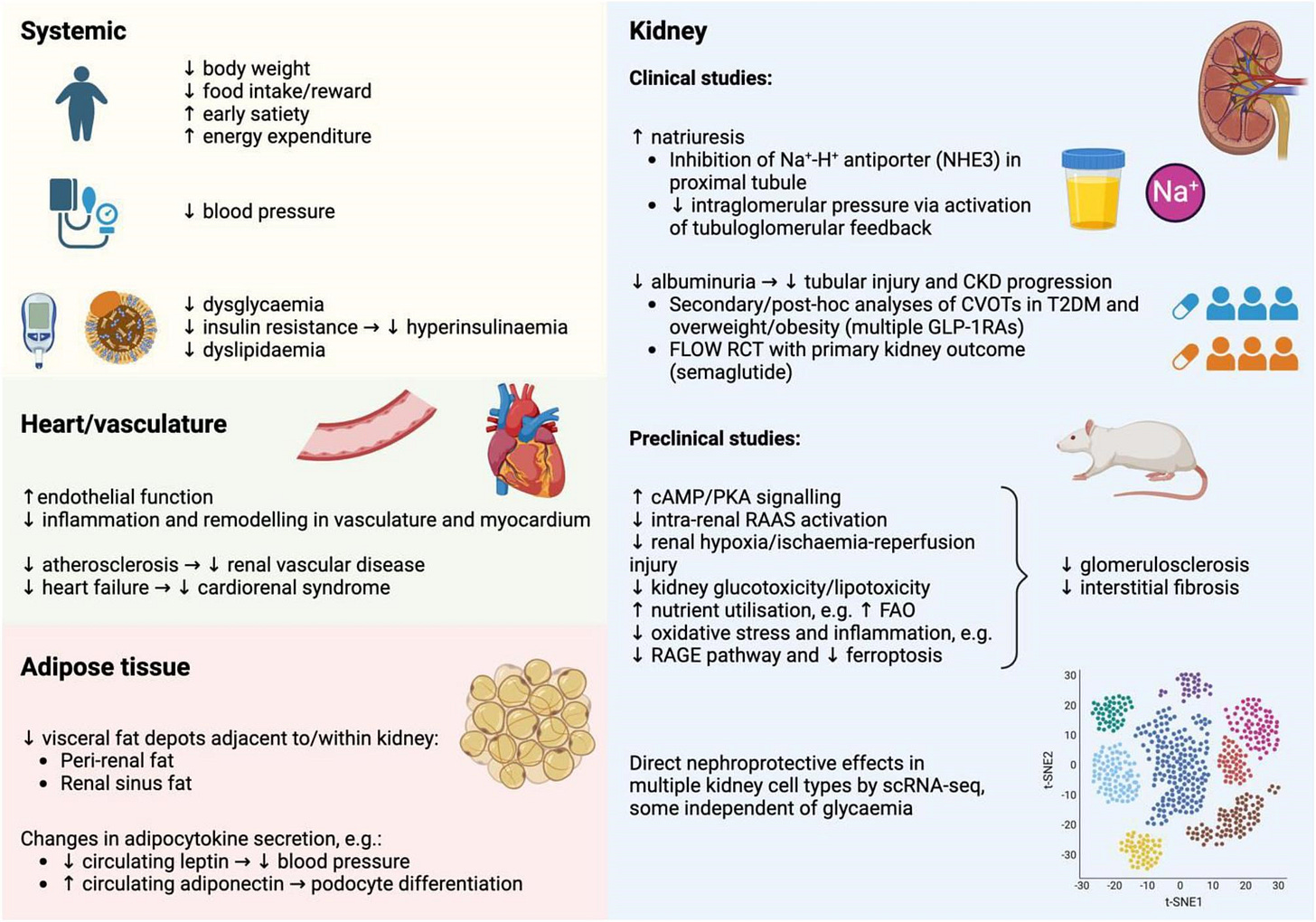
CARDIO-KIDNEY & OUTCOMES
A new Nephrology Dialysis Transplantation review positions GLP-1 receptor agonists at the center of a CKM therapeutic shift, emphasizing how SGLT2 inhibitors, GLP-1RAs, and nonsteroidal MRAs are converging to address cardiovascular, kidney, and metabolic risk holistically. The authors foresee next-generation triple agonists (GLP-1–GIP–glucagon) expanding treatment potential while helping close gaps in health equity and cardiometabolic outcomes. (Nephrol Dial Transplant, h/t Davide Garrisi)
A value-based care model significantly slowed CKD progression, according to an American Journal of Managed Care study of 623 patients with stage 3b–4 disease. Median eGFR decline dropped by 77% in stage 3b and 65% in stage 4 after enrollment, and participants maintained higher kidney function over time. The findings highlight how coordinated, high-touch care can delay kidney failure and improve patient outcomes through proactive management. (AJMC, eAppendix, h/t Strive Team)
Researchers are on the brink of doing what was thought impossible two years ago: evaluating SGLT2 inhibitors in children with CKD. The EMPA-KIDNEY® Kids study aims to demonstrate both the feasibility and promise of expanding kidney-protective therapies to younger populations, signaling a milestone for pediatric nephrology and research. Dr. Oni and colleagues shared the study protocol in Athens last week. (LinkedIn, ClinicalTrials.gov, h/t Louise Oni)
Kidney organoids emerge as the next frontier in renal replacement therapy. In the closing State-of-the-Art Lecture at ESPN 2025, Melissa Little outlined how stem cell–derived kidney organoids are transforming disease modeling and advancing toward stem cell–based renal replacement therapy. The work signals a future beyond dialysis and transplantation, where bioengineered kidneys could redefine treatment for children and adults alike. (ESPN 2025, h/t Melissa Little)
Hypertensive kidney disease deaths have surged 48% since 1999, according to new data presented at the American Heart Association’s 2025 Hypertension Scientific Sessions. The CDC analysis found men, Black and Hispanic adults, and residents of Southern states had the highest mortality rates, with Black patients experiencing a threefold higher risk. Researchers emphasized the need for earlier, more aggressive blood pressure control and CKD screening in high-risk groups to prevent upstream kidney failure. (R&U News, h/t Paul Gordon)
Figure: Depression Among Kidney Disease Patients
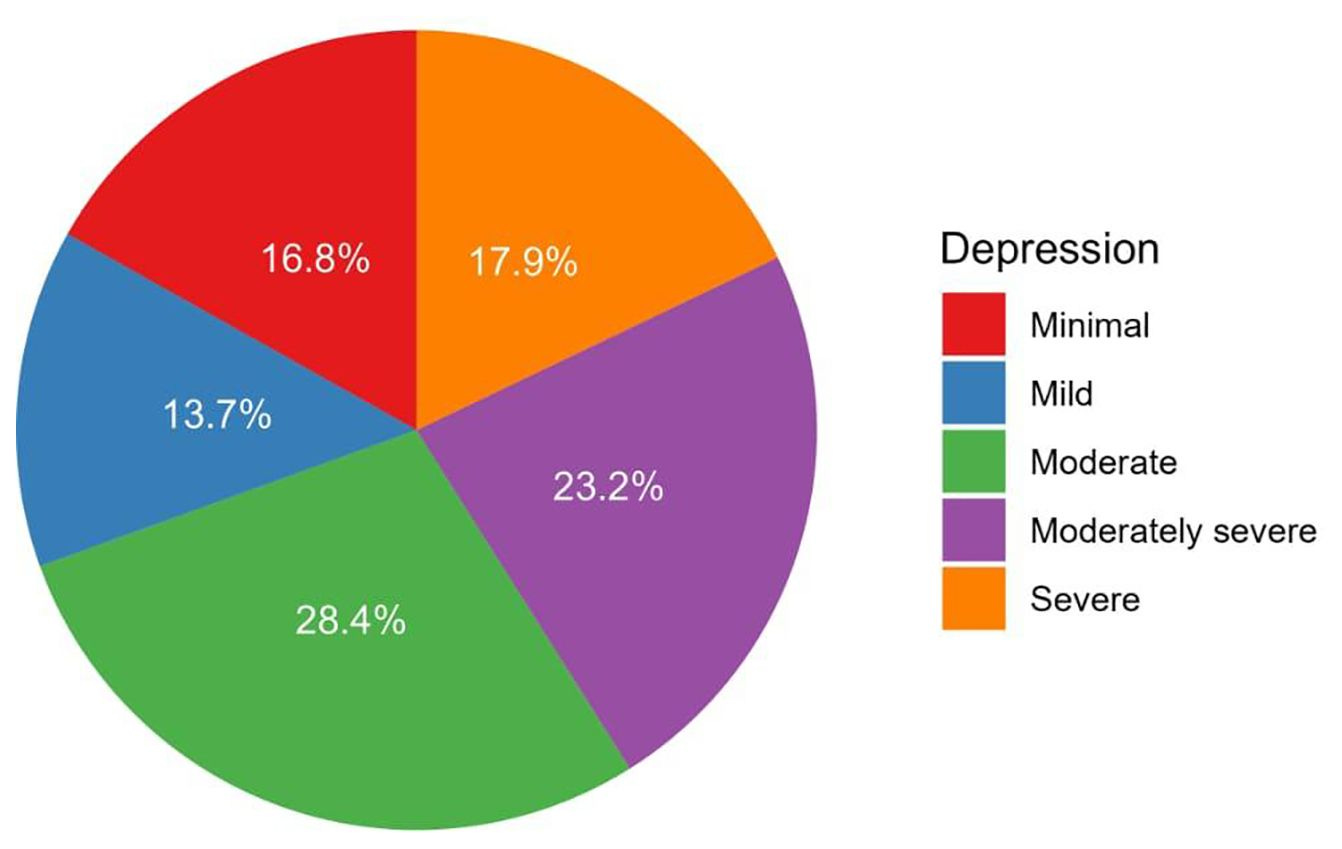
Depression remains a significant yet underrecognized burden among people with chronic kidney disease, according to a cross-sectional study from King Abdulaziz University Hospital in Jeddah, Saudi Arabia. Among 95 non-dialysis CKD patients, more than one in four met criteria for moderate depression, with symptoms most common in older adults, women, and those with multiple comorbidities. Higher depression scores were strongly linked to lower physical and mental quality-of-life measures, underscoring the interplay between mental health and chronic illness. The authors call for routine depression screening and integrated behavioral health support as essential components of kidney care. (Frontiers, h/t Paul Gordon)
Survey: Nephrology–dentistry coordination to improve CKD and ESKD outcomes [Link]. Led by Dr. Edgar Lerma and collaborators, the survey aims to explore opportunities to strengthen collaboration between nephrology and oral health professionals to enhance patient care and outcomes. Clinicians and researchers are encouraged to participate and share their perspectives. (Survey link here, h/t Edgar Lerma)
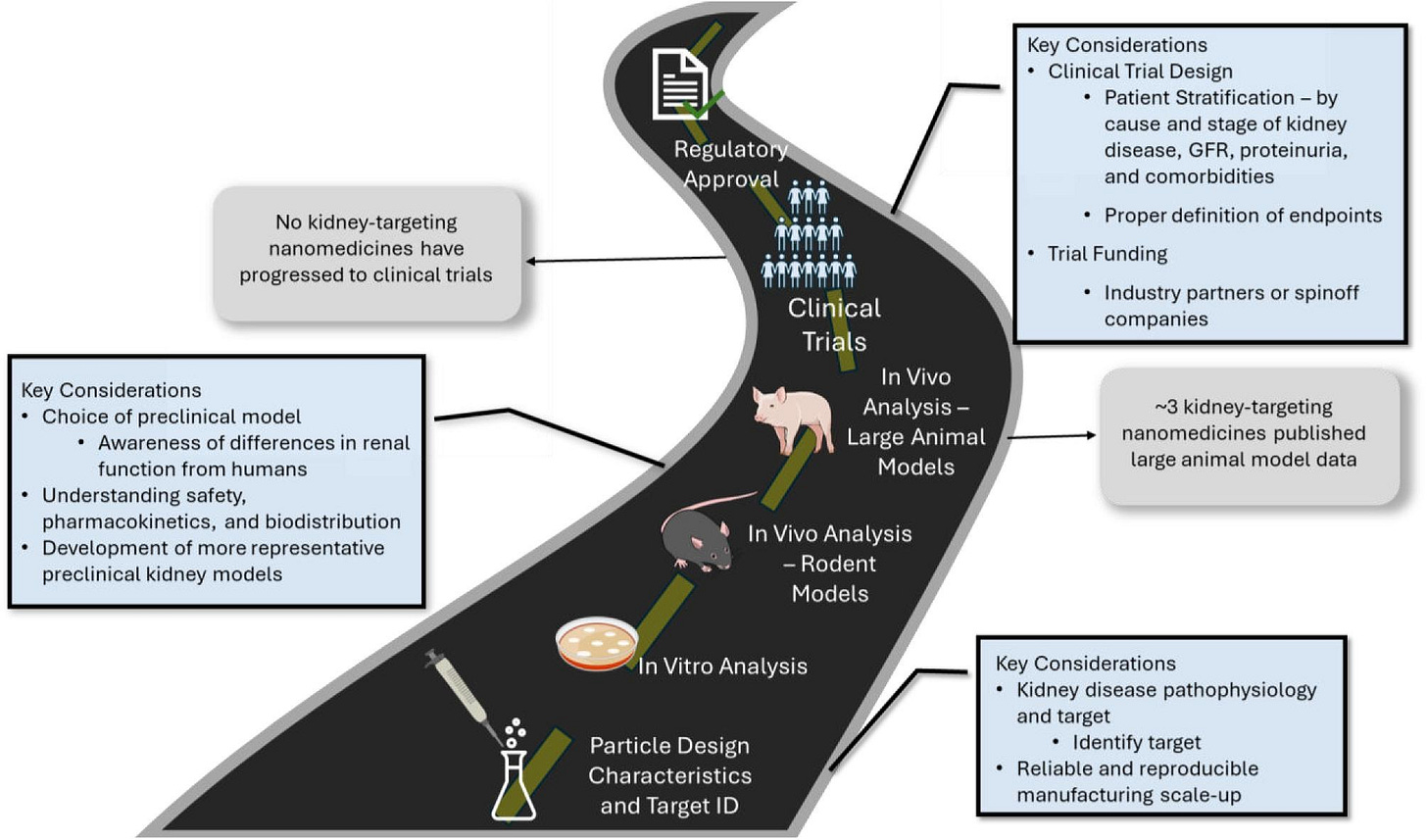
Nanomedicine is emerging as a promising approach for targeted drug delivery in kidney disease, according to a new Clinical Kidney Journal perspective from researchers at Columbia and Rockefeller Universities. The authors outline how nanoparticle size, shape, and surface chemistry can enable kidney-specific targeting, though no renal nanomedicines have yet reached clinical translation. They identify key barriers (e.g. limited preclinical models, manufacturing challenges, and trial design complexity) and call for closer collaboration between academia, industry, and regulators to advance kidney-targeted nanotherapies. Several candidates are now nearing clinical readiness, marking an inflection point for renal drug innovation. (Clinical Kidney Journal, h/t Ryan Williams)
TRANSPLANTATION
A new perspective calls for transplant candidates to play an active role in listing committee deliberations, arguing that greater transparency could help rebuild public trust in the U.S. transplant system. Writing in American Journal of Transplantation, Earnest Davis highlights how recent hearings, lawsuits, and media scrutiny have eroded confidence in organ allocation fairness. Citing new survey data showing most patients want participation in listing meetings, and believe it would enhance understanding and trust, Davis challenges the field to balance provider efficiency with patient autonomy. The piece urges transplant centers to embed shared decision-making into the evaluation process as a step toward restoring integrity and equity in organ transplantation. (American Journal of Transplantation, h/t Earnest Davis, PhD, MHSA)
AI and digital storytelling are helping potential living kidney donors make more informed decisions, according to new work from Dr. Amy Waterman and collaborators at the Digital Storytelling Project. The team’s Living Donation Storytelling Library now features over 200 multilingual videos that use real donor and recipient experiences to answer common fears and misconceptions. By pairing authentic narratives with AI-driven analysis of online search trends, the project seeks to demystify donation, address financial and logistical barriers, and empower more people to consider living kidney donation. (Houston Methodist, LinkedIn, h/t Mayra Almendarez)
The first living human pig kidney xenotransplantation has provided unprecedented physiological data, showing that a genetically modified porcine kidney can sustain essential metabolic and regulatory functions in a human host over 51 days. Published in Nature Communications, the study from Mass General Brigham documented normal waste excretion, electrolyte balance, and urine concentration, along with stable blood pressure and sodium handling. While mild metabolic adjustments required diuretic support, the findings demonstrate functional compatibility and highlight opportunities to optimize post-transplant management. The case establishes a crucial foundation for advancing xenotransplantation toward clinical reality. (Nature, h/t Marie-Camille Lafargue)
GENOMICS
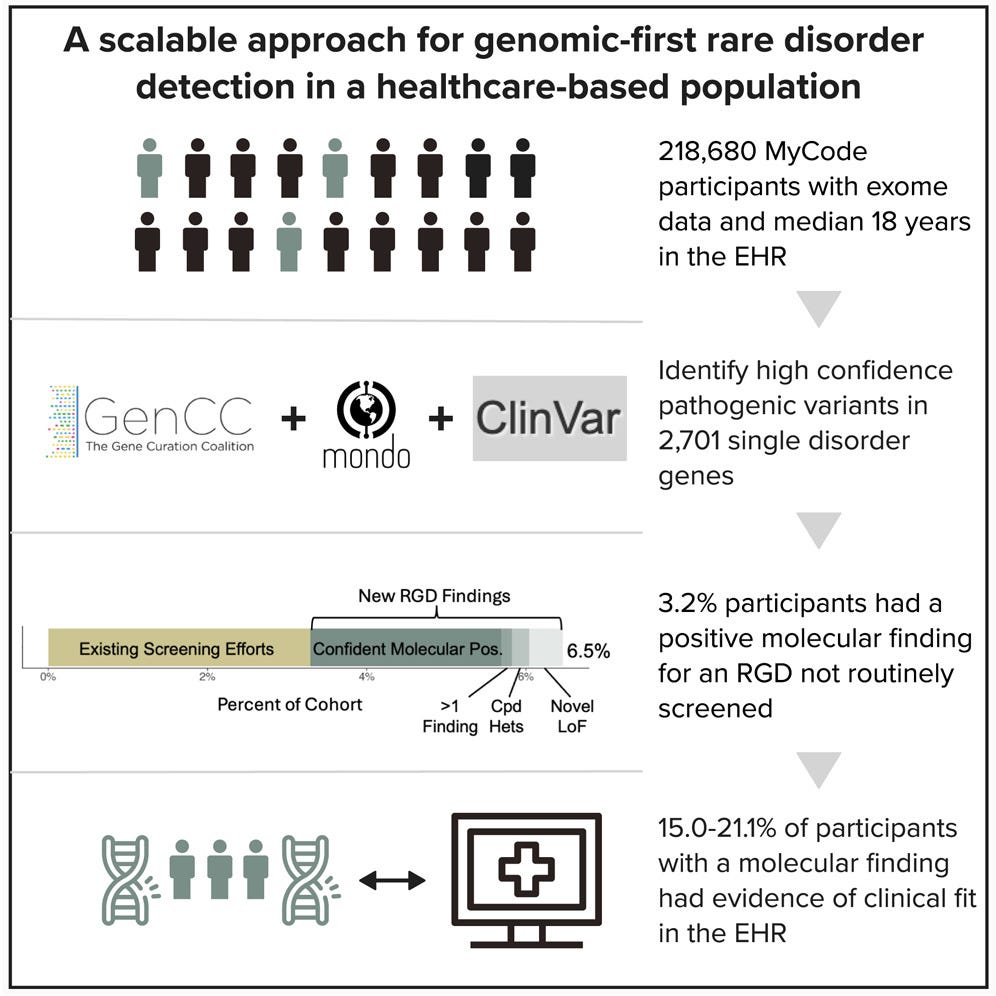
A genomic-first approach identified rare genetic diseases in 2.5% of participants, according to a AJHG study of more than 218,000 individuals in the Geisinger MyCode cohort. By screening for 2,700+ rare disorders beyond standard clinical testing, researchers uncovered high-confidence pathogenic variants often missed in routine care. Many participants had no prior diagnosis, underscoring the potential of genomics-first strategies to improve early detection, refine disease penetrance estimates, and guide proactive management. (AJHG, h/t Kyle Retterer)
A new AI system called CRISPR-GPT is redefining how researchers design and analyze gene-editing experiments. The large language model–based platform integrates domain expertise, retrieval tools, and human–AI collaboration to automate CRISPR experiment planning—from selecting systems and designing guide RNAs to analyzing results. In proof-of-concept studies, CRISPR-GPT successfully knocked out and activated target genes in human cell lines using CRISPR-Cas12a and dCas9 systems, validating its ability to function as an AI “co-pilot” for genome engineering. The work illustrates how large language models can bridge reasoning and biological design, enabling faster, more reproducible experimentation. (Nature, h/t John Milad)
A new study in Circulation mapped the earliest immune signatures in pig-to-human heart xenotransplantation, combining deep-learning pathology, transcriptomics, and histology to reveal how the human immune system recognizes porcine grafts. Led by Erwan Morgand and Alessia Giarraputo from the Paris Institute for Transplantation and Organ Regeneration (PITOR), in collaboration with NYU Langone and MGH, the team identified early microvascular inflammation arising de novo within the graft—an immune-driven process distinct from surgical effects. The findings establish a molecular and cellular framework for monitoring xenograft compatibility and inform future clinical trial design. (Circulation, Signals KOLs, h/t Erwan Morgand & Alessia Giarraputo)
“By combining all of this, we could essentially build a timeline, a sequence of biological events, based on serial biopsies. This kind of temporal mapping allows clinicians to understand not just what is happening, but when and why.” (Listen now)
SYSTEMS & POLICY
AI-enabled, community-integrated care models can substantially reduce healthcare utilization, according to a new study co-authored by Andrey Ostrovsky. The intervention, which combined predictive analytics with wraparound behavioral health and social support, was associated with a 52% reduction in emergency department visits and a 26% drop in inpatient admissions. Researchers say the approach demonstrates how scalable, data-driven care coordination can improve outcomes for safety-net populations while reducing costs. (JGIM, h/t Andrey Ostrovsky)
A JAMA Viewpoint weighs the significance the CMS Cell and Gene Therapy Access Model, a proposed federal framework for negotiating outcomes-based payment for high-cost genetic therapies.2 Authors Melissa Majerol, Aurelia Chaudhury, and colleagues highlight the potential for multi-stakeholder collaboration to improve affordability and access while addressing data-sharing and infrastructure gaps. The 33 states and participants represent 84% of Medicaid beneficiaries with sickle cell disease (SCD)—the first condition selected for this model. The analysis underscores how value-based frameworks could accelerate responsible adoption of next-generation therapeutics. (JAMA, h/t Melissa Majerol)
Medicare Part D access to GLP-1 therapies has sharply declined, according to new JAMA data highlighted by Brian Reid. The findings reveal that, while the drugs remain technically on formulary, administrative and prior-authorization barriers have made access increasingly difficult for people with diabetes—raising concerns about equity and continuity of care. Analysts warn the trend could blunt the public health benefits of these metabolic therapies. (JAMA, h/t Brian Reid)
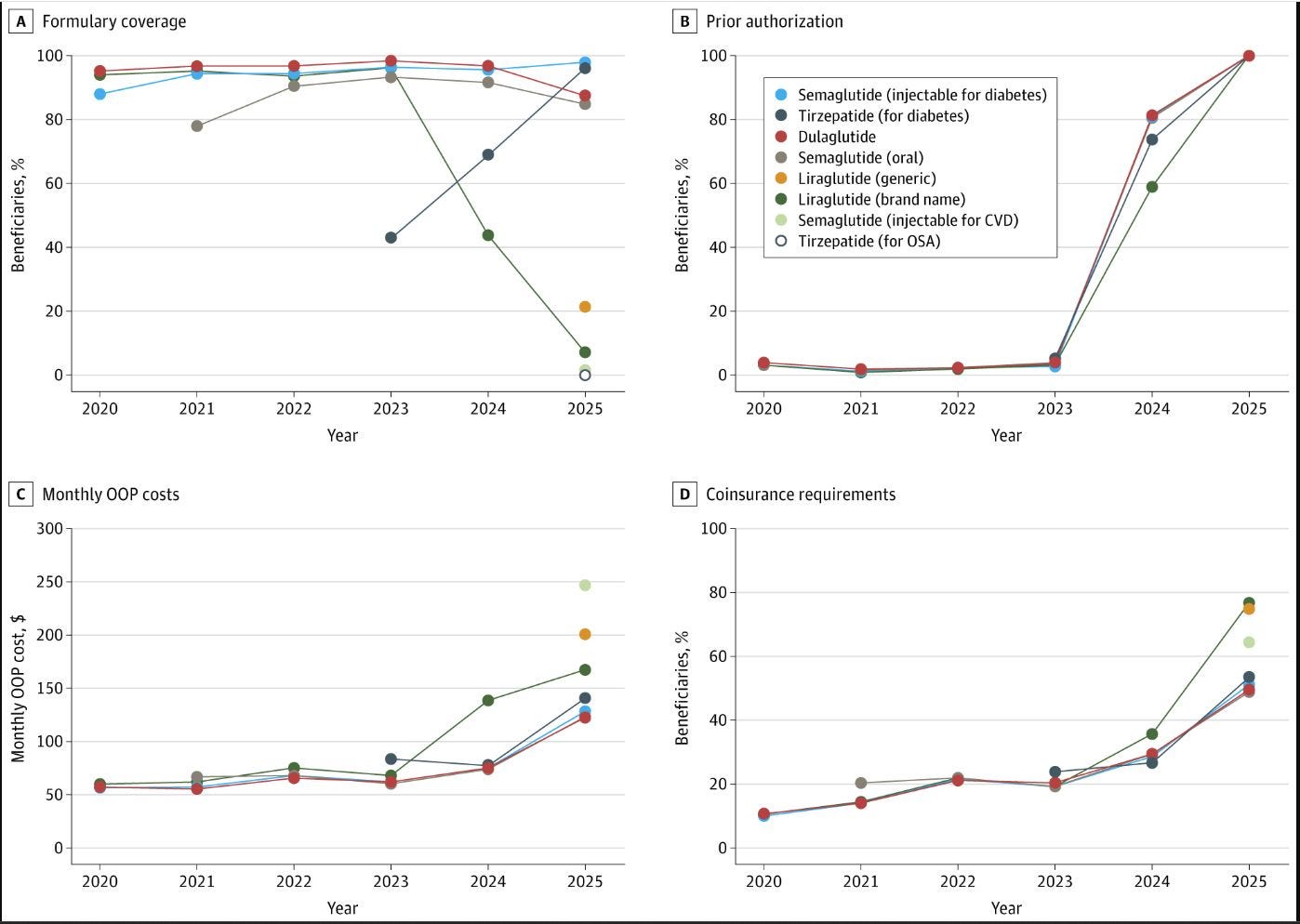
Artificial intelligence is rapidly expanding its footprint across nephrology, with experts from Kidney News highlighting applications in dialysis prediction, transplant decision support, pathology, and clinical education. Navdeep Tangri, Wisit Cheungpasitporn, and Len Usvyat described how algorithmic models are being deployed at scale to detect patient deterioration, automate documentation, and personalize care. The authors emphasized transparency, oversight, and clinician collaboration as essential guardrails as AI transitions from research to real-world implementation. (Kidney News, September 2025)
Critical thinking remains essential when interpreting medical research, cautions Gustavo Monnerat (Depty Editor at The Lancet). He shared a great carousel LinkedIn post and argued that even well-designed studies can mislead if readers ignore confounding, bias, or context. The commentary urges clinicians and policymakers to move beyond surface-level conclusions to assess whether results truly explain real-world outcomes. (LinkedIn, h/t Gustavo Monnerat)
Community
WINS & LEADERSHIP
Catherine Tritscher is celebrated for 30 years of service at Inserm and PITOR, recognized as a cornerstone of the Paris Institute for Transplantation and Organ Regeneration’s research and training community. A wonderful tribute by colleagues, who honored her mentorship, generosity, and legacy as she enters retirement. Congrats, Catherine! (LinkedIn, h/t Alexandre Loupy)
ASN reflects on two years of its unified journal portfolio, with JASN Editor Rajnish Mehrotra outlining how the society’s publications are fostering collaboration and advancing kidney science across disciplines. The update highlights growing global readership and a commitment to elevating diverse research voices. (Kidney News, h/t Edgar Lerma)
“I am most proud of the editorial teams that we have been able to assemble, tapping talent from around the world. Among the three journals, we have 56 editors (EICs, deputy editors, associate editors, and patient voice editors), 21 junior associate editors, 3 portfolio lead editors (communications, statistical, and visual abstract), 12 statistical reviewers, and 21 visual abstract editors.”
Mayo Clinic Arizona marked its 7,000th kidney transplant, a milestone reflecting decades of innovation and compassionate care. The achievement underscores the power of teamwork and patient trust in building a lasting transplant legacy. (LinkedIn, h/t Harini Chakkera)
COLLABORATIONS
Global leaders gathered in Chicago for the KDIGO Genetics in Kidney Health Summit, uniting nephrologists, genetic counselors, and patient advocates to improve access to genetic testing and education in rare kidney disease. The meeting advanced efforts to embed genomics into everyday kidney care worldwide. Read their 2022 report here.3 (LinkedIn, h/t Chang Alexander)
The PARASOL Project is expanding to include APOL1 kidney disease and primary membranous nephropathy, building on its work defining surrogate endpoints for FSGS. Coordinated by the International Society of Glomerular Disease with support from NephCure, ASN’s Kidney Health Initiative, and NKF, the new studies will evaluate proteinuria and anti-PLA2R antibodies as trial endpoints. The consortium aims to accelerate biomarker validation and regulatory alignment across rare glomerular diseases. (PARASOL Project / ISGD)
GlomCon Hawaii 2025 brought together global nephrology experts to explore the future of glomerular disease care, featuring sessions on IgA nephropathy, membranous nephropathy, lupus nephritis, and C3 glomerulopathy. Led by Ali Poyan Mehr and faculty, discussions highlighted new biomarkers, regenerative insights, and clinical trial design challenges shaping next-generation therapies. (Docwire News, h/t Joel Topf for the great recap)
Carna Health is screening 30,000 people for CKD across Cameroon, bringing digital diagnostics and population-based screening to hard-to-reach communities. The initiative demonstrates how technology can bridge access gaps and promote kidney health equity. (LinkedIn, h/t Elvis Ndansi)
Kenya’s Ministry of Health and kidney health advocates are integrating CKD into broader chronic disease programs, linking renal, cardiac, and metabolic care. The effort reflects a growing regional focus on early detection, multidisciplinary treatment, and policy alignment. (LinkedIn, h/t Stephen Mutiso)




POLICY & ADVOCACY
Former NFL star Nick Mangold seeks kidney donor, opens up about chronic kidney disease. The retired New York Jets and Ohio State standout revealed on social media that he is undergoing dialysis and in need of a kidney transplant due to a genetic defect diagnosed in 2006. With his family unable to donate, Mangold is asking fans—especially those with type O blood—to register as potential donors through New York-Presbyterian/Columbia’s Kidney Transplant Program. (ABC News)
Experts warn that CMS’s removal of key sociodemographic data from Form 2728 could undermine kidney equity efforts. In a Health Affairs article, Dinushika Mohottige, Tanjala Purnell, Morgan Reid, Keith Norris, and L. Ebony Boulware argue that the changes threaten decades of progress in tracking disparities and shaping equitable kidney policy. The authors note that the 2728 form has long been vital for documenting race, ethnicity, and social determinants of health at dialysis initiation—data essential for advancing the goals of the Advancing American Kidney Health initiative. (Health Affairs Forefront, NKF, LinkedIn, h/t Kevin Fowler)
NEJM perspective warns of risks in CMS WISeR model. Authors argue that expanding prior authorization to private contractors could lead to increased denials, delays in care, and higher administrative burdens for physicians. The piece urges CMS to adopt more transparent, patient-centered approaches to curb waste without restricting access. Author’s note: we heard a bit about this at the RPA AI Summit last weekend, which I wrote about here. (New England Journal of Medicine, h/t Rishi Wadhera and colleagues)
The ongoing value-based care debate enters the 4-pillars era. Christos Argyropoulos questioned whether value-based care models remain necessary as therapies like SGLT2 inhibitors, nsMRAs, GLP-1s, and RAAS blockers become standard, suggesting physicians could achieve similar outcomes through prescribing and adherence tools alone. In response, Dr. Katie Kwon noted that practical challenges such as EMR integration and administrative workload may make it difficult for many practices to participate without a VBC partner. Their exchange reflects how nephrology is reexamining care models in light of new therapies and real-world implementation hurdles. This topic is one Dr. Maru explored in a special section of Kidney News Dr. Kwon and I co-edited earlier this year. (LinkedIn, h/t Christos Argyropoulos and Katie Kwon)
Anna Kaltenboeck’s H1 2025 biopharma policy review tracks shifting incentives under MFN and global deal trends. Her team’s analysis shows R&D spending slowing amid policy uncertainty, Chinese firms increasing licensing activity, and U.S. pharma companies turning to direct-to-consumer models to adapt to the Trump administration’s pricing framework. The report signals a new era of selective innovation and pragmatic dealmaking. (ATI Advisory, LinkedIn, h/t Anna Kaltenboeck)
PERSPECTIVES
Rare & Genetic Kidney Disease Summit spotlights collaboration between patient foundations and pharma. Hosted in Boston, the event brought together leaders from Alport, NephCure, and CAKUT Foundations alongside industry partners to discuss biomarkers, trial models, and unmet needs in rare disease research. Conversations emphasized shared understanding of pathophysiology and the role of patient groups as key drivers of innovation. (LinkedIn, h/t Vincent Ko for sharing!)
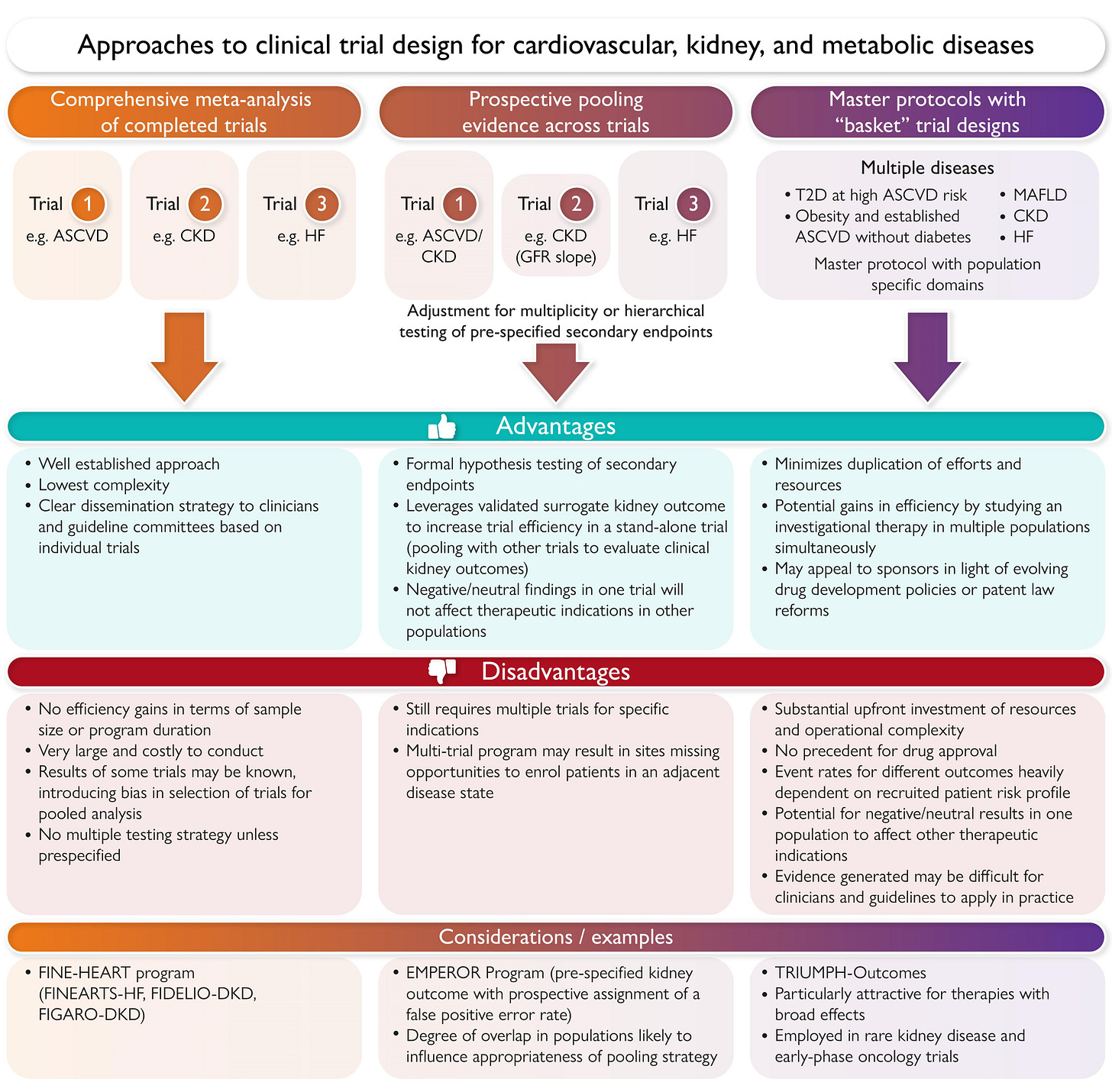
European Heart Journal introduces a new framework for cardio-kidney-metabolic trial design. Authors argue that traditional single-disease trials are no longer sufficient as multimorbidity becomes the norm. Their proposed approach integrates overlapping populations and basket-style designs to accelerate evidence generation across CKM conditions. (European Heart Journal, h/t Brendon Neuen and collaborators)
Agentic AI promises to reshape life sciences and MedTech. McKinsey’s new research finds that up to 85% of pharma and 80% of medtech workflows could be agent-enabled, potentially freeing 25–40% of staff capacity. The report highlights adoption challenges but envisions AI-human collaboration as a major driver of innovation and operational efficiency. (McKinsey, h/t Delphine Nain Zurkiya)
A 4,300-mile lung transplant journey from Maui showcases extraordinary teamwork and resilience. After months on life support due to respiratory failure, 58-year-old Michelle Ankele-Yamashita was airlifted from Honolulu to Nashville—becoming Vanderbilt Health’s first transplant patient from Hawaiʻi and one of the few ever transported long-distance while on ECMO. Her successful lung transplant, detailed in JTCVS Techniques, highlights the coordination and courage behind complex transplants and underscores the importance of preventive care, including flu vaccination. (Hawaii Public Radio, h/t Scientific Registry of Transplant Recipients)
Events Calendar
Countdown to a Cure NYC (New York, October 22)
ASN Kidney Week (Houston, November 5-9)
MedTech Strategist (San Diego, November 19-21)
VEITH Symposium (New York, November 18-22)
Int’l Transplantation Science (San Diego, Nov 16-19)
APCH (Lucknow, India, Nov 27-29)
Jobs
Nephrologist (Miami, FL) — Kaüna (Say hello!)
Director of Marketing — Renvio
Business Planning Strategist — Novartis
Director of Clinical Science — Travere
Director, Digital Engagement — Travere
Director, Strategy & Analysis — Otsuka
Director, Global Marketing (Neph) — Otsuka
Senior Assoc., Marekt Ops Support — Cityblock
Kidney Transplant Med Director — NYU Brooklyn
Sr. Manager, Corp Dev & Strategy — Ardelyx
Rare Disease Account Manager — Calliditas
SVP Customer Engagement — Akebia
Sr. Manager Federal Policy — Genentech
Data Engineer — Thermo Fisher Scientific
Business Development Director — Natera
…plus hundreds more at jobs.signalsfs.com
Work with us
Signals Group is expanding to support the growth of this community. Whether you’re looking to increase awareness for your work, prepare for your next milestone, or looking to enter a new market, we’d love to hear from you. We support mission-aligned organizations advancing kidney health.
Sponsor — Amplify your work to 20,000 kidney professionals
Grow — Partner with us to reach your next company milestones
Share — Tell us about your next milestones and let’s chat
This audio summary may include variations in pronunciation and is intended for informational purposes only. For complete accuracy and source attribution, please refer directly to the original written materials and cited sources. Always consult trusted references when interpreting medical or scientific content.
![Signals From [Space]](https://substackcdn.com/image/fetch/$s_!IXc-!,w_40,h_40,c_fill,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F9f7142a0-6602-495d-ab65-0e4c98cc67d4_450x450.png)
![Signals From [Space]](https://substackcdn.com/image/fetch/$s_!lBsj!,e_trim:10:white/e_trim:10:transparent/h_48,c_limit,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F0e0f61bc-e3f5-4f03-9c6e-5ca5da1fa095_1848x352.png)
![Signals From [Space]](https://substackcdn.com/image/fetch/$s_!NnOt!,w_144,h_144,c_fill,f_auto,q_auto:good,fl_progressive:steep,g_auto/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F688fc47b-7202-4a2e-b4f4-fea2b047ab1b_1500x1500.png)