When the first successful pig-to-human heart transplant made headlines in 2022, most people saw a medical milestone. For postdoc researchers Alessia Giarraputo and Erwan Morgand, they saw an opportunity to ask a deeper set of questions, starting with: what exactly happens inside the tissue when a human immune system meets a pig organ?
As co–first authors on a new landmark paper in Circulation, published last week and presented at the 18th international Congress in Geneva, the two researchers offer the most detailed look yet at the xeno-immune response in pig-to-human cardiac transplantation. Working between Paris’s PITOR Institute and collaborators at NYU Langone Health, their multimodal, machine learning-powered analysis sheds light on the cellular and molecular signatures that shape graft survival — insights that could help guide the next generation of clinical trials and the field of xenotransplantation.
What’s Inside:
From kidney to heart: lessons shaping the Circulation study
Mapping immune dynamics in pig-to-human transplants
Why clinical turnaround times matter for future trials
Inside PITOR’s global xeno research network
Highlights from the Geneva Congress and new data
What’s next for precision diagnostics and care
In this conversation, Alessia and Erwan discuss how their team combines deep-learning pathology, transcriptomics, and clinical workflows to map the immune frontier, and what it all means for the future of pig-to-human transplantation.1 We have several xeno pieces coming your way over the next few months. If you need quick refreshers on these topics, check out my recent articles on Lux Capital’s $191 million investment in eGenesis and the iBox scoring system. As always, I’d love to hear your take in the comments below. Thanks for being here with us.
What Happened
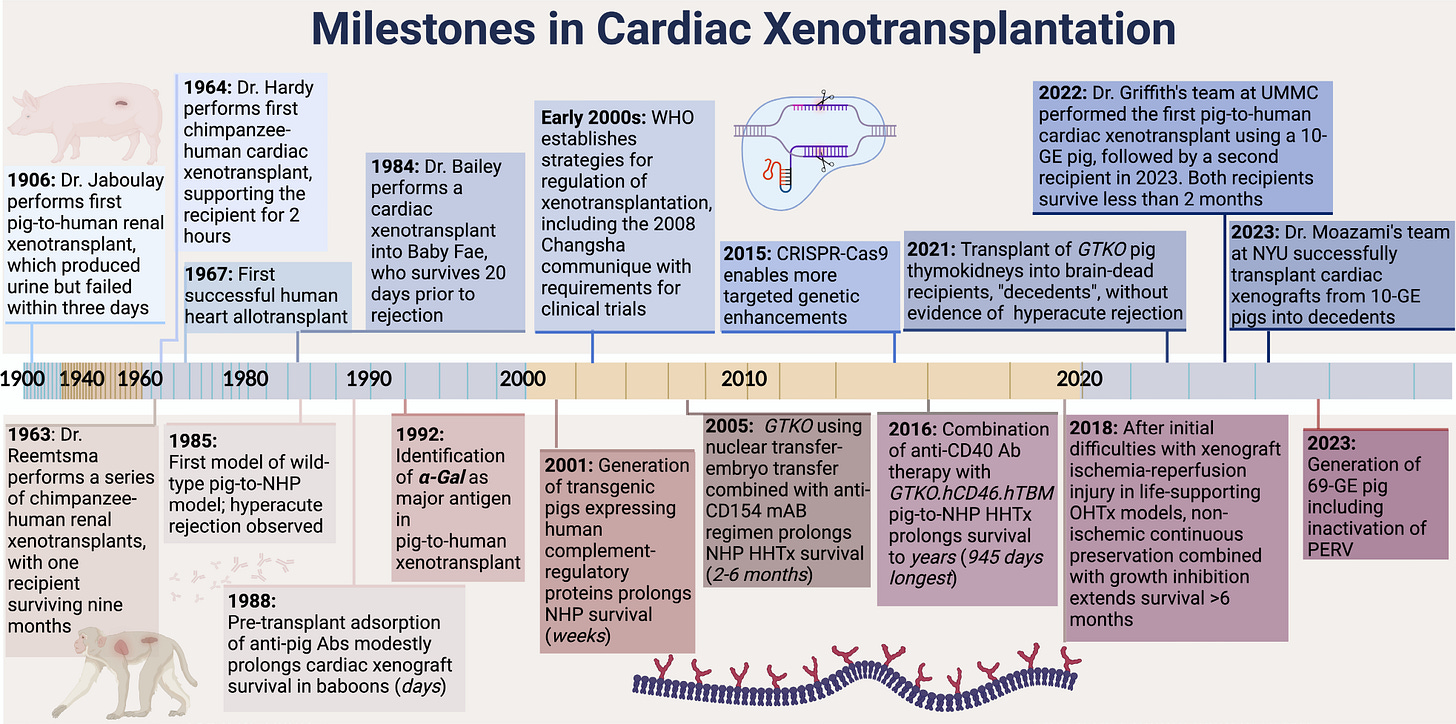
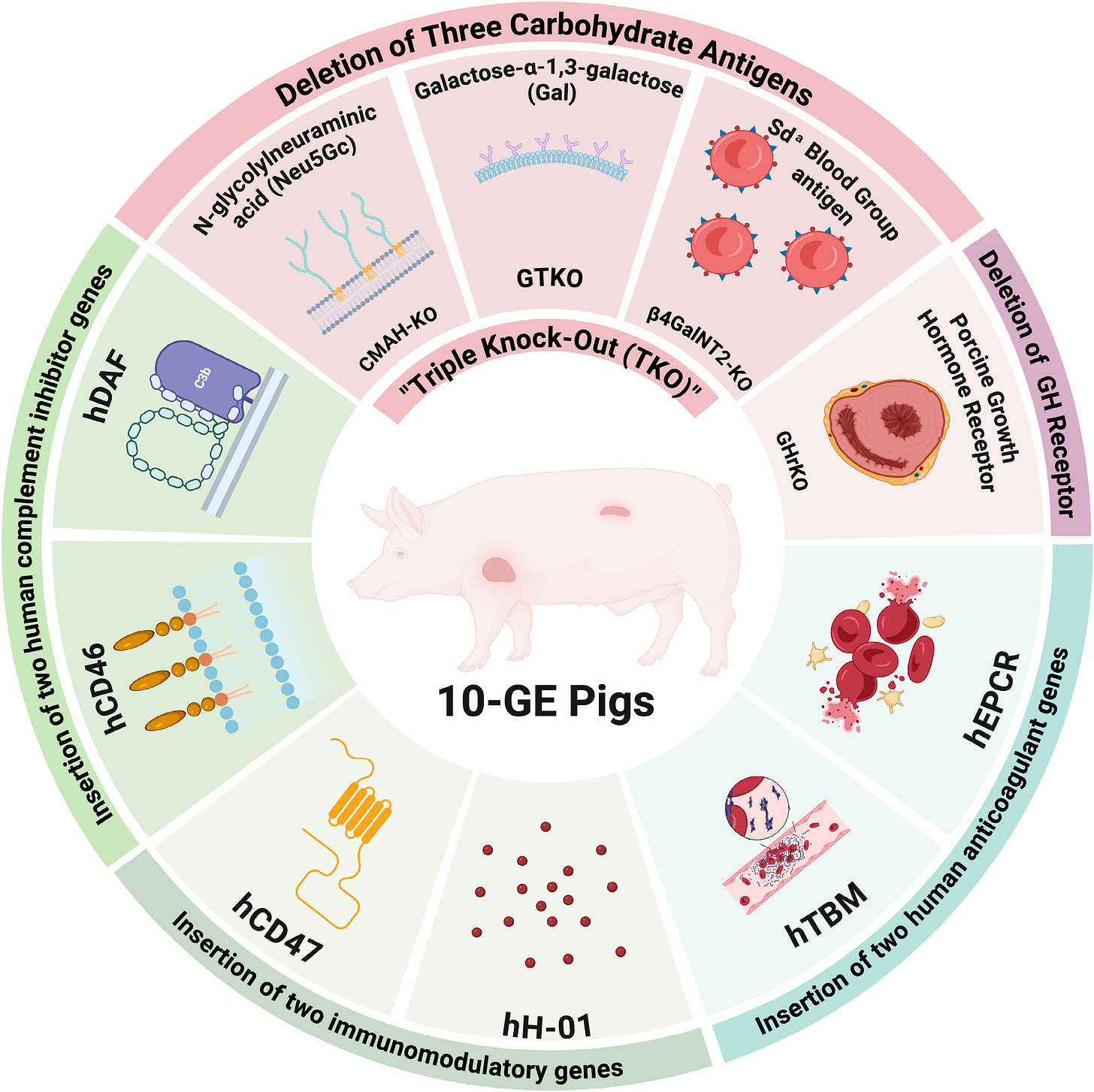
In a recent study, researchers transplanted two genetically engineered pig hearts, each carrying 10 targeted gene edits designed to prevent hyperacute rejection, into brain-dead human recipients at NYU Langone Health. Known as a decedent model, this approach enables scientists to study organ function and immune compatibility in a controlled human setting without clinical risk. At 66 hours after reperfusion, tissue analyses revealed early signs of a mild innate immune response.2

Using a multimodal diagnostic system that integrated histology, deep-learning image analyses, immuno-phenotyping, and bulk transcriptomics, the team was able to map the earliest phases of the xeno-immune response at unprecedented resolution. The findings mark an important step toward establishing new monitoring tools and immune signatures for future clinical xenotransplant trials.
Today’s conversation is with the two co–first authors of that study to hear firsthand what they learned, why it matters, and where the field is headed next.
Q&A with Erwan & Alessia
Welcome. Tell us a bit about yourselves and your work.
Alessia: I’m Alessia Giarraputo, a postdoctoral researcher at Massachusetts General Hospital and formerly at the Paris Institute for Transplantation and Organ Regeneration, or PITOR. My work focuses on xenotransplantation—specifically heart and kidney models—using multimodal systems to understand immune responses at a deeper level.
Erwan: And I’m Erwan Morgand, also a postdoc fellow at PITOR. I work on both xeno- (cross-species) and allo- (human-to-human) transplantation, mostly focusing on heart and kidney grafts. My background is in image analysis and transcriptomics, and I’m interested in integrating these data streams to map how the immune system reacts to transplanted organs.
Erwan, you’re joining us from Geneva at this year’s International Xeno Congress. Are you presenting findings from your latest paper?
Erwan: Thank you! Yes, the timing was perfect — our paper was published in Circulation the same day we presented it in Geneva. The study focuses on the xeno immune response in pig-to-human cardiac xenotransplantation. These experiments were performed in 2022 by the team at NYU Langone Health, led by Professor Robert Montgomery.
Our group at PITOR has been collaborating closely with them for several years on both heart and kidney xenografts. Once the procedures are done and initial analyses completed at NYU, they share samples with us so we can apply our integrative, systems-level approaches. These tools, originally developed for allotransplantation (human organs), help us explore what’s happening inside the porcine graft tissue and characterize the immune response in much more detail.
In short, this paper is about understanding how the human immune system recognizes and reacts to a pig heart graft, and what that means for improving future clinical xenotransplantation.
This study focused on the heart, but you’ve done similar work in kidneys before. How do those approaches compare?
Alessia: Exactly. Before this heart study, we used a similar framework for pig-to-human kidney xenotransplantation, also in collaboration with the NYU team. Those kidney experiments were incredibly informative because they allowed us to take serial biopsies over time and watch how the immune response evolved.

With the heart, we wanted to go a step further. Like Erwan mentioned, this is a very unique setting with a pig-to-human transplant, and we wanted to apply the most advanced tools available to capture what was really happening. So we adapted our multimodal system to the heart, knowing full well each organ has its own “story.”
That adaptation helped us identify something entirely new: a process of microvascular inflammation involving endothelial (blood vessel) cells that seemed to arise de novo — meaning it wasn’t caused by surgical or procedural factors, but rather by the immune response itself. Seeing that directly in the tissue and confirming it with transcriptomic data gave us strong evidence that something fundamental was happening at the molecular level.
These insights could one day help improve immunosuppressive treatment protocols and inform how future clinical xenotransplantation trials are designed.
So these findings could influence not just research but also eventual clinical practice?
Erwan: Yes, that’s one of the exciting aspects. One of the main strengths of this study is that every method we used is compatible with clinical turnaround times, meaning they could realistically be used in patient care.
As we move toward upcoming clinical xenotransplantation trials, especially those focused on kidneys, we’re now working with the NYU team to run these multimodal analyses in parallel with standard pathology. That way, clinicians can get deeper insights that might guide therapeutic decisions or inform patient management in real time.
Alessia, can you tell us more about the tools you used? What made this approach different from earlier studies?
Alessia: We started with the foundation we built in our kidney work at PITOR under Dr. Alexandre Loupy’s direction. Then we layered on more advanced, state-of-the-art methods — deep learning, for instance, to automate the quantification of immune cell infiltration in tissue samples. We also used transcriptomic analyses to see whether the transgenes (genes introduced into the pig to make the organ more compatible with humans) were being expressed, and which human genes were activated as part of the immune response.
By combining all of this, we could essentially build a timeline, a sequence of biological events, based on serial biopsies. This kind of temporal mapping allows clinicians to understand not just what is happening, but when and why.
The broader vision is to make these precision diagnostic tools adaptable for patient-specific contexts, especially as clinical xenotransplantation trials expand. Every patient’s immune system behaves differently, so having analytical systems that can evolve with the data is essential.
For readers who may not be familiar, what is PITOR, and how does it fit into this global collaboration?
Erwan: The Paris Institute for Transplantation and Organ Regeneration (PITOR) is a research institute led by Professor Alexandre Loupy. It brings together three major organizations: INSERM (France’s equivalent of the NIH), AP-HP (the consortium of Paris public hospitals), and Université Paris Cité.
The goal of PITOR is to combine clinical expertise, research, and data science under one roof. We work across organ systems, heart, kidney, liver, lung, and increasingly in xenotransplantation, where we’ve partnered with international teams like NYU Langone and the University of Maryland.
In fact, Alessia and I were also co-authors on an earlier Lancet study about pig-to-human kidney xenotransplantation, which laid the foundation for this new Circulation paper. We’ve also collaborated with Professor Muhammad Mohiuddin’s group in Maryland, who have performed living recipient heart xenotransplants. When you connect all these dots — NYU, Maryland, PITOR — you start to see a much clearer picture of the immune landscape across models and species.
Let’s talk about the Congress in Geneva. What stood out to you this week about where the field is headed?
Erwan: The energy is incredible. Xenotransplantation has been around for decades, but now it’s moving from theoretical to tangible clinical progress. There’s major momentum: new data, new collaborations, and a wave of pioneering teams entering the field.
We saw impressive updates from Mass General Hospital, where two patients have now lived three and eight months, respectively, with pig kidney transplants. That’s an extraordinary step forward. Alongside that, there’s growing research on immune modulation (how to better control or even reshape the xeno immune response), and on engineering improved pig models that could make future transplants safer and more sustainable.

Alessia, you’re now at Mass General. What perspective does that give you on this next chapter?
Alessia: It’s really inspiring. The xenotransplant field is evolving so fast, and what’s remarkable is the collaboration. Teams at NYU, Maryland, MGH, and PITOR are sharing information, comparing data, and learning from patients who are taking extraordinary steps to advance science and medicine.
At MGH, the current line of research builds directly on what we’ve discussed, translating lessons from the decedent models to living recipients. These patients are doing well, and every case teaches us something new.
The upcoming clinical trials will be transformative, both for patient access and for the science itself. Each transplant generates a wealth of information — immune, genetic, and transcriptomic — that can guide better monitoring and personalized care. We’re also adapting these “multi-omics” (integrated biological data) tools for the xeno setting, just as oncology once did when it pioneered precision medicine. Xeno-transplantation is now following that same path, and it’s bringing powerful new tools into the transplant field as a whole.3
Looking ahead, what are each of you focused on next?
Erwan: For me, it’s about kinetics, understanding how immune responses evolve over time and under different treatments. We now have long-term experiments that let us observe immune cell behavior and molecular changes across days, weeks, and months. Adding this temporal dimension makes the work more complex, but also more meaningful. It helps us identify the tipping points where tolerance might succeed or fail; insights that could shape the next generation of immunosuppressive therapies.
Alessia: And from my side, we’re seeing how essential integrated approaches have become. Single-point measurements can’t capture the full story. The future lies in combining histology, molecular data, and computational tools in a way that fits within clinical workflows.
Personalized diagnostics are no longer just theoretical; they’re becoming practical, with turnaround times and tissue requirements that match standard clinical care. If we can bridge that gap between research and bedside practice, xenotransplantation could redefine not only organ replacement but also how we think about precision medicine across fields.
Tim: That’s a great note to end on: the idea that xenotransplantation doesn’t just expand what’s possible, it changes how we approach the science itself. Congratulations again to both of you and your teams on the new paper and all your progress. Thanks for what you do, and for sharing with us on Signals.
My thanks to Alessia and Erwan for joining me to share their team’s work, and to Dr. Alexandre Loupy for making it happen. For more on PITOR and the Paris Transplant Group, visit their website here. I’ve also written extensively about transplant, including topics like Xenotransplant, the iBox Score, the End Kidney Deaths Act, and 25 Questions for the community. For more interviews and to learn how Signals Group is advancing global kidney health, visit us online at signalsfs.com.
Transcriptomics refers to the study of which genes are “turned on” or active in a given tissue.
Cardiac Xenotransplantation: Current State and Future Directions (Circulation, 2025)
![Signals From [Space]](https://substackcdn.com/image/fetch/$s_!IXc-!,w_40,h_40,c_fill,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F9f7142a0-6602-495d-ab65-0e4c98cc67d4_450x450.png)
![Signals From [Space]](https://substackcdn.com/image/fetch/$s_!lBsj!,e_trim:10:white/e_trim:10:transparent/h_48,c_limit,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F0e0f61bc-e3f5-4f03-9c6e-5ca5da1fa095_1848x352.png)