The last four to six weeks in kidney health feel different in a way that’s hard to overstate. Between the FDA’s accelerated approval for Voyxact, two landmark SGLT2 analyses, and a wave of new therapeutics pushing into late-stage development, it finally feels like nephrology is being treated as a major organ system, not an afterthought. And there’s a growing body of evidence to support that shift, including The Lancet’s latest data on CKD’s sharp rise in global mortality, the field’s first consensus on responsible AI use in nephrology, new hope in transplant immunology, Jesse Eisenberg stumbling into altruistic kidney donation, and louder calls for policy reform. The potential energy heading into 2026 is unlike anything I’ve felt in this space. In trying to hold the collective actions, memories, histories, and emotions of the kidneyverse, this update feels, dare I say… kinetic.
Kidney Week only reinforced this. I left more convinced than ever that the next decade will bring a true paradigm shift in how, when, and where we diagnose, manage, and treat kidney disease: earlier, more precisely, and with better tools. This update alone includes findings from multiple decade-long studies, each reshaping our sense of what works and where we go next, including culturally tailored care, SGLT2 therapy, and long-term graft survival.
And candidly, curating and writing these updates feels different, too. Synthesizing all of this in one place makes the trendline obvious: things are tipping forward. My gut says stay the course, double down, and keep pushing. Because the momentum is real, and the window to drive meaningful change is opening. Let’s open the doors while we’re at it.1
We also released three new pieces this month, including part one of our VBC report, my interview with Jullie Hoggan, and an update on rural kidney care, each reinforcing the shifts underway.
Have tips or feedback? Hit reply or drop us a note here.
Reading time: 35 minutes
What’s Inside
News: FDA IgAN milestone, 70k-patient SGLT2 data, Lancet CKD burden jump, ASN AI framework, KCAPA introduced in Congress, NYT on altruistic donation, decade-long Navigate-Kidney CHW trial…
Research: New KTR ML risk models, high-DSA graft survival insights, pig-to-human xenotransplant findings, AI in drug development, rare-disease AI, JASN phosphorous guidance, CKM coordinated-care review, urine dipstick screening…
Community: ANNA leadership updates, Unity Health green dialysis, Panoramic poster redesign, Australia advocacy summit, transplant milestones, miR-17 ADPKD discussion, AKI “sixth vital sign,” RPM policy debate…
Jobs: Kaüna, Natera, ProKidney, Mozarc, CorMedix, Calliditas, Yale, Stanford, Intermountain, Paragonix, Open Philanthropy, Oura, NIH + dozens more on jobs.signalsfs.com…
This issue is made possible by Renalytix. Renalytix is an artificial intelligence-enabled in vitro diagnostics company, focused on optimizing clinical management of kidney disease to drive improved patient outcomes. Renalytix has received FDA approval and Medicare reimbursement for kidneyintelX.dkd which is offered commercially in the United States. Learn more about the test below, and watch my recent interview with Chief Medical Officer and Lab Director, Michael Donovan.
News

People are talking about new global data showing that chronic kidney disease is rising sharply, driven by diabetes, hypertension, obesity, and aging. The most recent Lancet analysis estimates that 788 million adults worldwide had CKD in 2023 and identifies it as the ninth leading cause of death. A New York Times piece underscores how CKD often goes undetected despite simple screening tests and effective kidney-protective therapies. Only a third of people with diabetes and a small fraction with hypertension receive recommended urine and blood tests, leaving most cases unrecognized until late stages. The NYT coverage (among several others) signals growing mainstream attention to a condition long overlooked in public health. Keep it coming, we need more lumens shining bright on the pitfalls and progress happening in kidney disease. Don’t let up. (The New York Times, h/t Nina Agrawal)
DRUG & THERAPY R&D
FDA granted accelerated approval to Voyxact (sibeprenlimab), the first APRIL-targeting biologic for IgA nephropathy. The once-monthly self-injection is the first biologic ever approved for IgAN and the first anti-APRIL agent to reach the market in a fast-crowding field. In the Phase 3 Visionary trial, Voyxact showed a placebo-adjusted 51 percent reduction in proteinuria at nine months and 54.3 percent at one year, on top of standard care that included ACE inhibitors, ARBs, and SGLT2 inhibitors. Unlike previous accelerated approvals, the FDA did not restrict eligibility by UPCR level, reflecting confidence in the class’s eGFR trajectory and offering broader access for patients at risk of progression. Competition in the APRIL and BAFF/APRIL space is rising quickly, with programs from Novartis, Vertex, Vor Bio, Vera, and others advancing behind it. (Fierce Biotech, h/t Vlado Perkovic)
ProKidney released full Phase II data for its autologous cell therapy rilparencel, showing some of the strongest signals yet that kidney function decline may be slowed through regenerative approaches. The therapy uses a patient’s own kidney cells, expanded from a small biopsy to billions of cells that express repair-oriented markers before being reinjected into both kidneys. In the REGEN-007 trial, rilparencel improved the annual eGFR slope by 4.6 mL/min/1.73 m², a 78 percent improvement over historical expectations, with no serious treatment-related adverse events and a safety profile comparable to a routine biopsy. ProKidney, which holds RMAT designation, is now running the global Phase III PROACT 1 study using eGFR slope as the primary endpoint, with an interim readout expected in mid-2027.2 CEO Bruce Culleton says the goal is to delay or prevent dialysis by activating the kidney’s innate repair pathways, a concept rooted in two decades of work dating back to early tissue-engineering breakthroughs. If Phase III results confirm the benefit, rilparencel could become the first approved regenerative cell therapy for CKD. (Inside Precision Medicine, h/t Bruce Culleton)
The Vertex Foundation committed $3 million to ASN to launch EKCITE, a new program focused on training the kidney workforce in immune-mediated therapies. The initiative, which ASN will match with an additional $2 million, aims to prepare nephrologists and kidney health professionals to deliver emerging treatments across a spectrum of kidney diseases. Training will cover immune monitoring, early infection identification, and management of complications and side effects—areas that are becoming central as a new generation of therapies reaches the clinic. ASN leaders say nephrology is experiencing a “renaissance in drug development,” and the goal is to ensure clinicians are ready to implement these advances. The Foundation describes EKCITE as an investment in the people who will carry these innovations to patients. (Press release, h/t Prabir Roy-Chaudhury)
Eledon released Phase 2 BESTOW results showing that tegoprubart may offer strong kidney function with fewer toxicities than tacrolimus. In de novo kidney transplant recipients who stayed on therapy for one year, the mean 12-month eGFR was about 69 mL/min/1.73 m², with a safety profile that substantially reduced the metabolic, neurologic, and cardiovascular side effects often seen with tacrolimus. Investigators highlighted lower rates of new-onset diabetes, tremor, and delayed graft function, arguing that tegoprubart may represent a next-generation option as the program moves into Phase 3. Karin Hehenberger, a physician-scientist and transplant recipient who recently wrote a guest post on this topic, shared that the results align with her lived experience of tacrolimus toxicity and called the data encouraging for patients seeking safer long-term immunosuppression. (Press release, h/t Karin Hehenberger)
Bayer reported positive Phase 3 FINE-ONE results showing that finerenone significantly reduced albuminuria in people with CKD caused by type 1 diabetes. The trial met its primary endpoint, with meaningful reductions in UACR compared with placebo, a signal that has been linked to better long-term outcomes in earlier type 2 diabetes studies. Finerenone is already approved for CKD in type 2 diabetes, and today’s data suggest it may also lower the risk of kidney disease progression, kidney failure, and cardiovascular complications in T1D, a group with very limited treatment options. There were no new safety concerns, and Bayer plans to seek regulatory review to expand the drug’s indication. Breakthrough T1D called the results a major step forward, noting that this is the first therapy in three decades to show a clear benefit for CKD in people with type 1 diabetes. (Press release, Breakthrough T1D,h/t Richard Pratley)
ACUTE & PEDIATRIC CARE
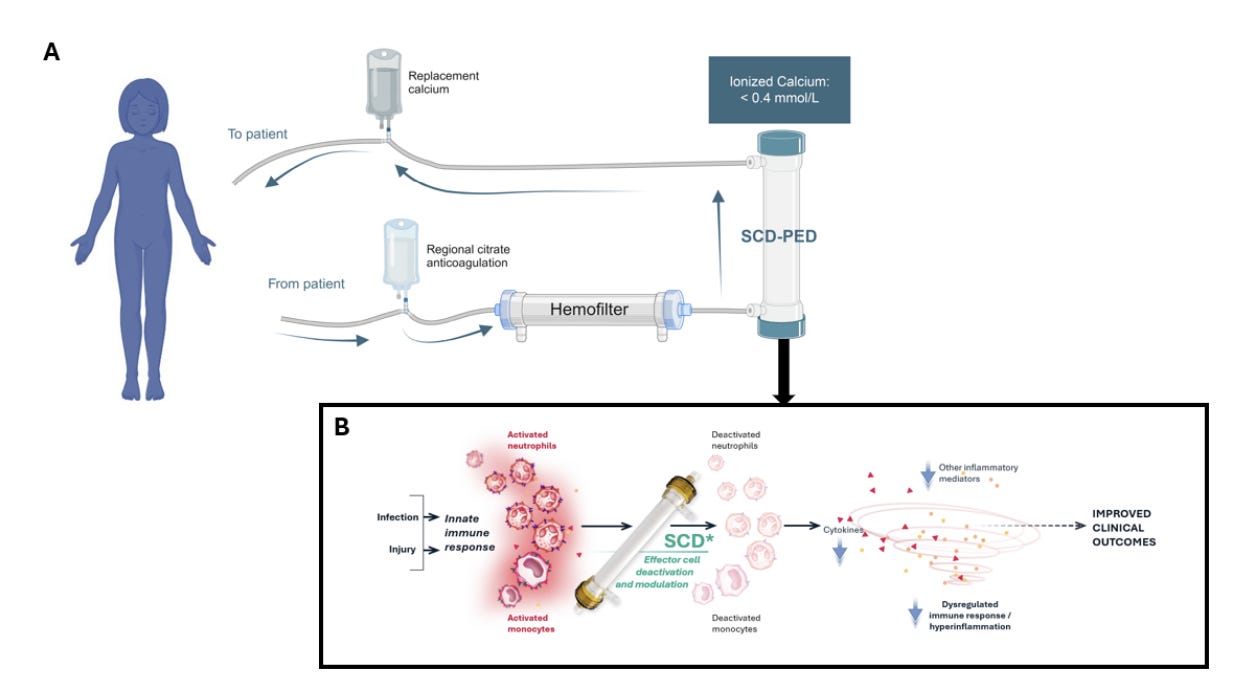
SeaStar Medical shared new data showing that its Selective Cytopheretic Device (SCD) may improve outcomes for critically ill children with AKI receiving CRRT. In a comparison of two prospective SCD studies with patients from the WE-ROCK registry, children treated with SCD had shorter CRRT duration, shorter ICU stays, and higher survival to ICU discharge or Day 60. The analysis showed a greater than 99 percent probability of improved survival with SCD, and outcomes were even stronger in septic patients, who saw 100 percent survival and faster recovery. The therapy targets activated neutrophils and monocytes in an extracorporeal circuit to reduce hyperinflammation, a key driver of organ injury in pediatric MODS, though the authors note that larger prospective studies are still needed. SeaStar Medical also announced the appointment of Michael Messinger as Chief Financial Officer, adding more than 25 years of financial and operational leadership as the company expands commercial efforts for its QUELIMMUNE (SCD-PED) therapy. (Blood Purification and press release, h/t Sai Iyer)
New results from the CANSCAN feasibility trial show that Vexev’s VxWave system can reliably perform semiautonomous vascular mapping inside dialysis clinics. The multicenter study, conducted with U.S. Renal Care and presented at Kidney Week, scanned 115 patients with stage 4 or 5 CKD. Investigators reported a 94 percent scan completion rate, 100 percent data adequacy, and successful determination of feasible vascular access options in 98 percent of patients. The trial also identified anatomy patterns that may disadvantage certain groups, including increased arterial calcification and smaller distal vessels in patients with diabetes and fewer available veins in women. Vexev and clinicians say these findings support bringing vascular mapping directly into the dialysis unit to speed access planning and improve workflow. (Endovascular Today, h/t Eamonn Colley)
TRANSPLANTATION
NIH is seeking input to shape the next decade of transplant clinical trials. NIAID released an RFI to refine its CTOT-CA program, which builds on two decades of CTOT consortia work spanning ~200 sites, ~7,000 participants, and 33 multi-center studies. The initiative has grown from biomarker discovery to early- and late-phase trials of drugs, biologics, and cell therapies, supported by a mature national infrastructure. NIAID is asking stakeholders to identify the biggest transplant challenges ahead, advise on how CTOT can stay nimble amid shifting allocation policies and enrollment hurdles, and propose ways to expand use of existing CTOT data and samples. Responses are due January 19, 2026. (NIH – NIAID, h/t Stuart Sweet)
United Therapeutics completed the first UKidney™ transplant in its EXPAND clinical trial, a major step forward for xenotransplantation. The procedure at NYU Langone used a 10-gene–edited pig kidney engineered to improve immune compatibility and reduce rejection risk. United Therapeutics described the milestone as a move toward giving ESRD patients, especially those unlikely to receive a human donor organ, an alternative to lifelong dialysis. NYU’s Robert Montgomery called it a transformative moment for the thousands waiting for a kidney who may not survive long enough to receive one. EXPAND is a multicenter, open-label, “phaseless” Phase 1/2/3 study designed to support a future BLA, with 24-week primary follow-up and lifelong monitoring for kidney function and zoonotic safety. (Press release, h/t Macey Levan )
ASTS and TransMedics are supporting a biosensor platform for real-time monitoring during normothermic machine perfusion. Dr. Michelle Nguyen’s team at Mayo Clinic Arizona is building technology to track key biomarkers while organs are perfused, allowing transplant teams to make more informed decisions. (LinkedIn, h/t American Society of Transplant Surgeons)
Paragonix Technologies launched a national distribution fleet to improve access to its organ preservation technologies and clinical support services. The program creates a dedicated supply chain to ensure hospitals, OPOs, and transplant centers can receive Paragonix devices and on-demand clinical support reliably and on time. The first vehicles will operate in Florida and California, expanding access in two of the most active transplant regions. Paragonix says the fleet will give partners a single-source solution for preservation products, surgical and donor coordination services, and air and ground logistics. The company now supports more than 18,000 transplant cases and serves over 260 U.S. transplant centers, representing more than 80 percent of programs nationwide. (Press release, h/t Paragonix Technologies)
Research

CARE DELIVERY
A ten-year research effort just showed us that culturally tailored support can measurably improve dialysis care for Latino patients, thanks to a landmark randomized trial led by Dr. Lilia Cervantes at CU Anschutz. In the Navigate-Kidney trial, Cervantes and colleagues demonstrated that a CHW-led intervention meaningfully reduced interdialytic weight gain and strengthened patient confidence and self-management — progress driven by trust, cultural alignment, and real partnership. Patients described the program as transformative, and results were published across leading journals, including JAMA Internal Medicine and JAMA Network Open (CU News, h/t Lily Cervantes).
Two SMART-C companion studies published in JAMA show SGLT2 inhibitors deliver consistent kidney protection across 70,000+ patients with diabetes, CKD, or heart failure. The SMART-C consortium found a 35–40% reduction in CKD progression, slower eGFR decline across all albuminuria levels, including stage 4 CKD and patients with little or no albuminuria, and meaningful reductions in hospitalizations and mortality. The absolute benefits were similar across disease states and far outweighed potential harms, reinforcing SGLT2 inhibitors as foundational therapy across the CKD spectrum (JAMA 1, JAMA 2, SMART-C, h/t Brendon Neuen).
A new systematic review in Mayo Clinic Proceedings highlights how coordinated care models can improve outcomes for people living with overlapping cardiovascular, kidney, and metabolic conditions. Across 22 international studies, ranging from multidisciplinary clinics to pharmacist-led programs and patient education models, coordinated CKM care was linked to higher patient satisfaction, fewer complications, better visit adherence, and lower costs, especially when virtual care and telehealth were included. Despite variation in program design, the evidence points to meaningful benefits for patients navigating multiple chronic conditions. Glenda Roberts and colleagues led this important work, underscoring the need for standardized reporting and best-practice models to guide the next generation of CKM care (Mayo Clinic Proc, h/t Glenda Roberts).
Is It Time for Population-Based Urine Dipstick Screening? A new analysis in Kidney International Reports argues that low-cost urine dipsticks may offer a practical population-level tool for early CKD detection. With CKD now affecting ~850 million people worldwide and projected to become the #5 cause of years-of-life-lost by 2040, the authors note that inexpensive screening could help identify albuminuria much earlier — especially now that proven therapies can delay kidney failure by decades. Given the silent progression of CKD and the rising global economic burden, the paper calls for renewed attention to dipstick testing as a scalable, $0.20-per-strip first step toward earlier intervention (KI Reports, h/t Sayna Norouzi)
AI, DATA & DECISION MAKING

Reimbursement failures are blocking the impact of health AI, according to a new NEJM AI perspective led by Eric Topol and colleagues. The authors argue that despite major advances in AI, health care remains one of the least transformed sectors because economic and regulatory frameworks have not kept pace. Even FDA-cleared AI tools struggle to gain traction due to outdated reimbursement pathways, CPT code bottlenecks, and fragmented incentives. The paper outlines specific reforms to align payment models with AI cost structures and reduce integration overhead, while also calling for a forward-looking regulatory framework for generative AI. The authors frame the issue as an ethical one: failing to modernize payment systems prevents tools that could reduce clinician workload, shorten wait times, and improve patient care from reaching patients. (NEJM AI, h/t Eric Topol)
New Nature Review outlines a global roadmap for point-of-care kidney diagnostics in low-resource settings. A team led by Priyanka Singh, Kate Bramham, June Fabian, Robert Kalyesubula, Valerie Luyckx and Cathy Haldane argues that point-of-care tools are essential for closing the diagnostic gap in low- and middle-income countries, where most CKD goes undetected until late stages. The review highlights that simple tests like dipsticks and portable creatinine analyzers can dramatically expand early detection, but only if paired with reliable validation, strong supply chains, integration into health systems, and sustainable financing. The authors lay out four priorities—validation, implementation, health-economic evaluation and equity—to turn the recent WHO CKD resolution from aspiration into impact. They stress that POC diagnostics are “not a luxury, but a global health necessity.” (Nature Reviews, h/t Robert Kalyesubula)
A major JASN statement outlines foundational principles for the responsible use of AI in kidney care. Convened by the American Society of Nephrology, the multidisciplinary AI Workgroup emphasizes patient benefit, clinician oversight, transparency, and equity as core guardrails for integrating AI into CKD, AKI, dialysis, and transplant workflows. The review highlights both the promise of predictive and generative AI and the risks posed by poor data quality, biased models, and weak clinical integration. It offers practical guidance for nephrologists looking to adopt AI tools while keeping the physician firmly “in the loop” to protect patient safety. (JASN,h/t Len Usvyat)
Figure: Guiding principles of the ASN workgroup on responsible AI and kidney disease
Two companion NEJM AI papers highlight the FDA’s emerging framework for responsible use of AI in drug development, including rare diseases. The first paper summarizes an FDA–CTTI workshop outlining guiding principles for integrating AI across the drug and biologic development lifecycle, emphasizing the need for clear policy, technical standards, and guardrails to enable safe, scalable adoption. The second addresses one of the field’s hardest problems: applying AI in rare and ultrarare diseases, where data are scarce, heterogeneous and geographically dispersed. The authors describe strategies such as advanced analytics, individual-level modeling, synthetic data generation, and centralized data infrastructure to overcome these barriers. Together, the papers offer an early blueprint for credible, patient-centered AI in regulatory science. (NEJM AI, h/t Tala Fakhouri)
A new study in Applied Human Factors… maps practical solutions to close the privacy gap in digital health research. UC San Diego researchers used a co-design framework to tackle one of the biggest challenges in modern research: the complexity and opacity of third-party privacy policies that govern data from wearables, apps, and digital tools. In a workshop with IRB members, researchers and past participants, the team analyzed a real-world device policy and created six prototype solutions, including a policy-scoring tool, personalized data-risk profiles, an interactive consent interface, and an IRB support dashboard. Stakeholders prioritized simplicity, transparency, and participant-centered design. The authors argue that without clearer privacy communication, both risk assessment and informed consent are compromised. (AHFE, h/t Karandeep Singh)
A new feasibility study in Applied Clinical Informatics shows that guided, mobile critical-care algorithms can strengthen rapid response performance across physician training levels. Researchers at Mass General Brigham tested five digital pathways for common deterioration scenarios and found consistently high ratings for reduced cognitive load, improved confidence, and better standardization of care. New PGY-2 residents reported more difficulty navigating the tool than attendings, highlighting the need for UX design that supports clinicians under stress. The work was done in collaboration with Avo, a company behind several tech-enabled clinical workflows in kidney care, suggests that well-designed digital “co-pilots” can elevate acute care performance and bridge experience gaps in high-stakes situations. (ACI, LinkedIn, h/t Jared Conley)
TRANSPLANTATION
A new Transplantation study provides 15-year outcome data for kidney recipients with high levels of preformed donor-specific antibodies (MFI > 3000). Researchers found substantially lower long-term graft survival in DSA-positive recipients — 47.6% at 15 years versus 72.8% in matched DSA-negative controls. Rates of antibody-mediated rejection were also significantly higher in the DSA group, while T-cell–mediated rejection rates were similar. Notably, patient survival did not differ between groups despite the graft survival gap, suggesting that aggressive immunosuppression and close monitoring can still support long-term outcomes. (Transplantation, h/t Olivier Aubert)
A new JACC: Advances study shows that machine-learning models can match or modestly outperform traditional tools for predicting cardiovascular risk in kidney transplant recipients. Researchers analyzed more than 1,000 clinical, laboratory, imaging, and procedural features from 518 transplant recipients and tested multiple ML approaches. The best performing approach reached an AUROC of 0.72 — slightly higher than logistic regression and significantly better than the widely used Soveri score.3 Key predictors included coronary artery disease, dialysis duration, left atrial strain, and ventricular volume measures. The authors note that while differences were not statistically significant, the findings show that ML-based risk prediction is feasible and may offer earlier, more individualized cardiovascular risk assessment in a population where standard calculators routinely underperform. (JACC: Advances, h/t Mina Mehanni)
A new Nature study offers the clearest look yet at how a pig kidney performs in a human body. NYU and international collaborators transplanted a gene-edited pig kidney into a brain-dead human and maintained kidney function for 61 days. The organ produced urine, kept electrolytes stable, and didn’t require dialysis — a major milestone showing that even a minimally modified pig kidney can support human physiology for weeks. Midway through the study, the kidney showed signs of rejection driven by human antibodies and T cells. When doctors treated the rejection with standard transplant therapies, kidney function recovered. The study shows both how far xenotransplantation has come and the immune barriers that still need to be solved before pig kidneys can be used in living patients. (Nature, h/t Alexandre Loupy)
A new NEJM publication reports 10-year follow-up data showing the long-term benefits of hypothermic machine perfusion using the LifePort Kidney Transporter. The study extends the landmark 2009 Moers et al. trial and shows sustained improvements in graft survival compared with static cold storage. For expanded-criteria donor kidneys, 10-year graft survival reached ~70 percent with LifePort compared with ~60 percent for static cold storage. DCD kidneys showed a similar advantage at ~81 percent versus ~76 percent. (NEJM, h/t Tom Daulton)
A new review in Transplant International compares donor travel with organ shipment in kidney exchange programs. Analyzing 105 studies across four domains (cold ischemia time, logistics, donor and recipient perspectives, and professional views) the authors note that most countries now favor shipping the kidney rather than moving the donor. Longer cold ischemia times can increase delayed graft function but generally do not affect long-term outcomes. Donor travel, meanwhile, is often emotionally and financially burdensome and linked to lower willingness to participate in exchange. The review concludes that programs should tailor their approach to local realities while prioritizing strategies that lower barriers to participation, whether by improving organ-shipping logistics or reducing the burden on donors who must travel. (Transplant Int, h/t Thierry Berney)
Advocating for rehabilitation as standard transplant care. A new CJASN perspective revisits Belding Scribner’s original vision for kidney replacement therapy: not just survival, but a return to independence and full participation in life. The authors argue that kidney transplant recipients still lack access to structured, reimbursed rehabilitation programs—support that cardiac and pulmonary patients routinely receive. They call for integrating pre- and post-transplant rehab into routine care to improve outcomes, reduce costs, and help patients thrive after transplant. (LinkedIn, h/t Amber Paulus and Kevin Fowler)
THERAPEUTICS
A new JCI review highlights that GLP-1–based medicines do far more than lower blood sugar or support weight loss. Beyond their well-known metabolic effects, drugs like semaglutide and tirzepatide appear to have direct anti-inflammatory actions that may help reduce complications across multiple chronic conditions, including heart disease, liver disease, kidney disease and sleep apnea. Emerging “multi-hormone” therapies may amplify these benefits by targeting additional pathways involved in inflammation. The piece underscores that some of the broader health improvements seen with GLP-1 therapies may come from immune and inflammation effects separate from weight loss. (JCI, h/t Eric Topol)
A large NEJM trial finds that high-dose fish-oil supplementation can meaningfully reduce cardiovascular risk in patients on hemodialysis. Among 1,228 participants, daily n−3 fatty acids cut serious cardiovascular events by 43 percent compared with placebo, with consistent benefits across cardiac death, myocardial infarction, stroke and peripheral vascular disease. Safety and adherence were similar between groups. The findings suggest fish oil may offer a rare preventive option in a population with few effective therapies. (NEJM, h/t Kam Kalantar-Zadeh)
A new JAMA study analyzed 740 high-engagement social media posts about three major drug classes, revealing just how widespread undisclosed pharmaceutical promotion has become. More than 57 million views were generated by posts where 80% showed signs of influencer-style promotion without required disclosures, and only one in three mentioned risks alongside benefits. Pharmaceutical companies accounted for less than 5% of the content, highlighting how much drug marketing now flows through patient and lifestyle creators rather than official channels. Junelle Speller noted that these gaps raise real concerns for patient safety and reinforce the need for stronger oversight of digital drug promotion. (JAMA, h/t Junelle Speller)
Community
INDUSTRY
Paragonix supports a historic transplant milestone in Poland, where clinicians performed two heart transplants in a single day — exactly 40 years after Prof. Zbigniew Religa’s landmark surgery — and credited the SherpaPak system for enabling the achievement (LinkedIn, h/t Szymon Kawalko).
Tonix and MGH advance a Phase 2 trial of TNX-1500 for transplant rejection, launching a collaboration to study an Fc-modified anti-CD40L antibody designed to prevent kidney transplant rejection (Press release).
Figure: Diffusion and Convection Process
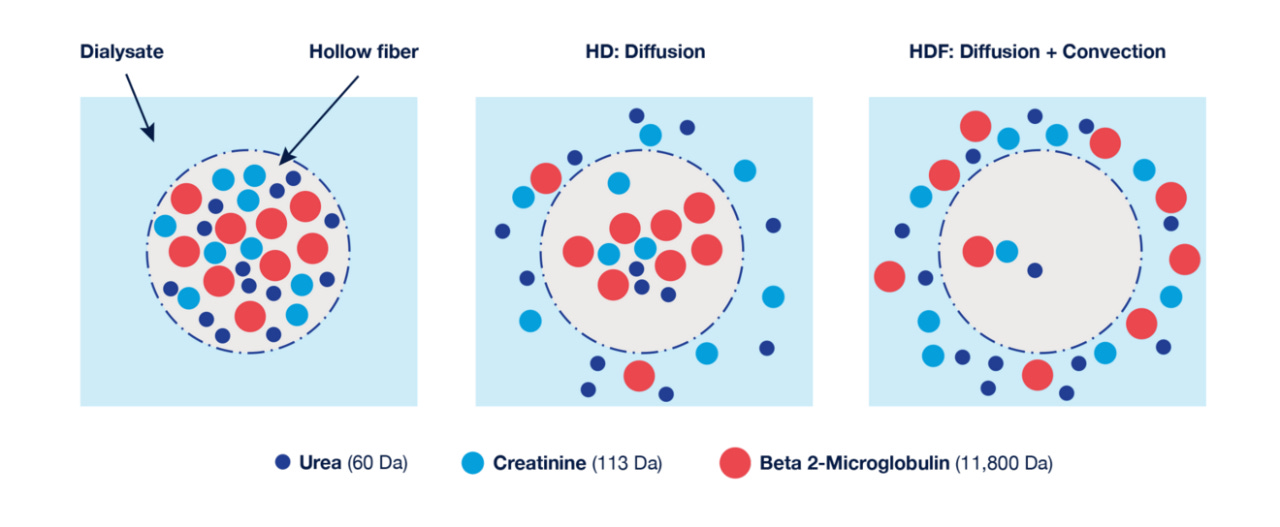
High-volume HDF launches across Southern California, with Fresenius rolling out the therapy region-wide in January, with the aim of bringing the comfort and long-term benefits of HvHDF seen internationally (LinkedIn).
Todd Dunn makes the case that the kidney is the only vital organ without a vital sign, and it’s time to modernize how we detect and manage AKI. Despite AKI affecting up to 40% of patients after cardiac procedures, clinicians still rely on 1930s-era gravity bags to monitor urine output, leaving kidney distress invisible until it’s too late. He highlights how digital systems like Accuryn can finally treat urine output as a continuous, actionable vital sign and bring kidney monitoring into the same century as the heart and lungs (eCQI, LinkedIn).
POLICY & ADVOCACY
Mahesh Krishnan argues it’s time to modernize Medicare’s kidney policies, noting in Chief Healthcare Executive that the ESRD bundle has effectively “frozen” innovation since 2011 and calling the Kidney Care Access Protection Act a needed pathway to restore reimbursement, support new technologies, and improve long-term access to dialysis care (CHE, h/t Mahesh)4
KCP welcomes new momentum behind modernizing Medicare’s kidney benefit. and Representatives Carol Miller and Terri Sewell introduced the bipartisan Kidney Care Access Protection Act (KCAPA) to stabilize reimbursement, expand education access, and create a sustainable pathway for new therapies (miller.house.gov, press release, h/t KCP).
The American Nephrology Nurses Association announced new leadership, appointing Lou Ann Leary as Executive Director and Celess Tyrell as Chief of Staff, marking a major transition for the nation’s leading nephrology nursing organization. Leary brings more than 30 years of service to ANNA, including roles supporting chapters, guiding national programs, and serving as Interim Executive Director. Tyrell, a 20-year ANNA veteran, has led major digital and educational initiatives, from online learning to volunteer engagement (Press release, h/t Rebecca Harvie)
Ariel Dora Stern shares new evidence on RPM policy tensions, showing via Health Affairs that RPM expanded access and supported higher-burden patients, and arguing that the solution is smarter guardrails—not blunt coverage cuts like UnitedHealthcare’s 2026 policy (Health Affairs, STAT).
AHRQ details $200M in primary care research investments, with Paulius Mui highlighting new datasets, workforce grants, and quality-improvement projects, while raising questions about how future investments should be targeted (LinkedIn).
Rajesh Aggarwal highlights academic medical centers (AMCs) venture investment trends, summarizing a JAMA Internal Medicine study showing $24B invested across nearly 700 companies and arguing that AMC-backed VC can accelerate innovation aligned with clinical and operational needs. AMCs have increasingly established their own venture capital (VC) funds, sourced from endowments, royalties, licensing fees, and philanthropy, to invest in health care companies (JAMA, LinkedIn, h/t Rajesh).
RESEARCH PERSPECTIVES
Panoramic Health profiled Dr. Katie Kwon’s push to reinvent the modern research poster, arguing that traditional text-heavy designs bury the real story behind the science. Her approach treats posters as conversation starters—stripped down, visually clear, and anchored around a single message that draws people in. Kwon’s iterative, design-first process has already stood out at Kidney Week, where her creative layouts sparked strong engagement and showed how better storytelling can elevate clinical research. It’s a reminder that great science needs great communication to drive collaboration and impact. (Panoramic Blog, h/t Katie Kwon) Note: in case you missed it, Dr. Kwon wrote a fantastic guest post in June, detailing how “first past the post” billing rules make it harder to deliver coordinated care for patients with complex needs. Read it here.
James Weatherall outlines how AI is reshaping drug discovery and development at AstraZeneca. He shares how the company’s modality-rich pipeline and interoperable data foundation now enable AI tools to drive smarter, faster R&D. Think generative design platforms that cut small-molecule identification time in half, or multi-agent systems that compress literature review and trial design from months to hours (LinkedIn, h/t James Weatherall).
Sachin Jain calls for innovation aimed at ultra-high-acuity patients, arguing in Forbes that value-based care has focused too narrowly on moderate-risk chronic disease while overlooking the small population of ultra-complex, ultra-cost patients who need new models the most (Forbes).
Unity Health Toronto highlights decades-long leadership in sustainable dialysis, showcasing how its nephrology and clinical engineering teams replaced single-use acid jugs with a centralized delivery system that cuts plastic waste, lowers greenhouse gas emissions, and improves staff workflow (LinkedIn, YouTube, h/t Jeffrey Perl).
COMMUNITY VOICES

Kidney health leaders across Australia held their annual Centre for Research Excellence in Kidney Health workshop, organized by Nicki Scholes-Robertson (Founder, Rural Kidney Association NSW) and Germaine Wong, bringing together more than 100 patients, caregivers, researchers, and clinicians from Adelaide, Brisbane, Sydney, and Melbourne. Lily Cervantes presented on community-partnered research and advocacy and helped lead one of the workshop’s action-oriented breakout groups, where participants explored how patient voice and co-design can drive real healthcare policy change. The two-day event highlighted the power of community-building, shared leadership, and implementation science to advance kidney health equity (LinkedIn, h/t Lily Cervantes).
“How do you plan to incorporate gene therapy when/if it becomes available for clinical practice?” That question from Xavier Vela Parada frames a new Kidney News piece showing how ADPKD research is shifting toward rescuing the patient’s own wild-type PKD1/2 allele rather than adding a new gene. It’s early, but if upcoming phase 3 data hold, this allele-rescue strategy could reshape how gene-targeted therapies are delivered in ADPKD (Kidney News, LinkedIn, h/t Xavier Vela Parada).
Updated guidance from JASN outlines new strategies to help people with CKD reduce hidden potassium and phosphorus additives in their diets, offering a clearer, tiered framework for clinicians and dietitians. The workgroup recommends basic strategies, like limiting processed foods and encouraging balanced meals, that any clinician can support, while advanced and intensive strategies focus on directly identifying and avoiding additives through label reading, substitutions, and dietitian-led counseling. These approaches aim to reduce additive exposure without overwhelming patients, matching guidance to each person’s food literacy and support needs. (JASN; Purdue Nutrition Science, h/t Annabel Biruete).
Iqra Aftab captures the “real digital health gap,” contrasting AI hype with the daily realities of clinic life (e.g. crashing EMRs, faxed notes, handwritten labs, staff schedules) and reminding innovators why so much digital health fails at the point of care. It’s an important reality check that technology + capital alone cannot touch many of the gaps and -isms that make healthcare human (LinkedIn).
Piedmont Transplant Institute sets a new state record with liver transplants #176 and #177, celebrating a milestone made possible by close coordination across surgeons, coordinators, ICU staff, donor partners, and the broader care team. The program’s Executive Director Leah Phillips Baker noted the significance of this achievement for expanding access and delivering high-quality transplant care across the state of Georgia (LinkedIn, h/t Leah Phillips Baker).
We’d love to hear from you. What questions and comments do you have from this month’s recap? What stood out to you, and what else should we know?
Events Calendar
APCN X TSN 2025 (Taipei City, December 5-7)
LYSNT Conference (Tripoli, Libya, December 5-7)
HASIN Challenges Conference (Athens, December 7)
Global CVCT Forum (Washington, DC, Dec 8-10)
NKF Roundtable: IgAN (Iowa City, Dec 10)
AdvaMed Med Device Webinar (Dec 11 at 2pm ET)
JPM Healthcare Conference (SF, January 12-15, 2026)
NephCure Support Groups (Ongoing)
Jobs
Nephrologist (Miami, FL) — Kaüna (Say hello!)
Director of Marketing — Renvio
Research Scientist — Oura
Director, HealthTech Works — Yale University
Director, Medical Education Strategy — Otsuka
People Operations Manager — Abridge
Medical Science Liaison — Novo Nordisk
Manager, Care Enablement — Diverge Health
Senior Technical Services Specialist — Mozarc
Senior Manager, Clinical Operations — CorMedix
Medical Director (Nephrology) — Calliditas
Senior Director, Digital Medicine — Pfizer
Senior Product Dev Engineer — Paragonix
Director, Business Development — Natera
Investment Analyst Intern — Intermountain Health
…plus hundreds more at jobs.signalsfs.com
Work with us
Signals Group is expanding to support the growth of this community. Whether you’re looking to increase awareness for your work, prepare for your next milestone, or looking to enter a new market, we’d love to hear from you. We support mission-aligned organizations advancing kidney health.
Learn — Discover hundreds of articles & interviews in our Data Room
Share — See how Signals can help you reach your next milestones
Sponsor — See why our 2026 sponsorships are filling up fast
This audio summary may include variations in pronunciation and is intended for informational purposes only. For complete accuracy and source attribution, please refer directly to the original written materials and cited sources. Always consult trusted references when interpreting medical or scientific content.
RMAT stands for Regenerative Medicine Advanced Therapy, which is an FDA designation that helps speed up the development and review of certain regenerative medicine products for serious conditions. To qualify, a product must be a regenerative medicine therapy (like cell or gene therapy), show preliminary evidence of potentially treating a serious disease, and have the potential to address unmet medical needs. (fda.gov)
A historical risk calculator using 8 different features from Soveri et al was used as well as a reference.
S.2730 - Kidney Care Access Protection Act (congress.gov)
![Signals From [Space]](https://substackcdn.com/image/fetch/$s_!IXc-!,w_40,h_40,c_fill,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F9f7142a0-6602-495d-ab65-0e4c98cc67d4_450x450.png)
![Signals From [Space]](https://substackcdn.com/image/fetch/$s_!lBsj!,e_trim:10:white/e_trim:10:transparent/h_48,c_limit,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F0e0f61bc-e3f5-4f03-9c6e-5ca5da1fa095_1848x352.png)
![Signals From [Space]](https://substackcdn.com/image/fetch/$s_!NnOt!,w_144,h_144,c_fill,f_auto,q_auto:good,fl_progressive:steep,g_auto/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F688fc47b-7202-4a2e-b4f4-fea2b047ab1b_1500x1500.png)