Most people with early-stage kidney disease don’t know they have it. For many, the first sign comes too late—when kidney function has already fallen dramatically. Michael Donovan, who serves as Chief Medical Officer and Lab Director at Renalytix, believes that can change.
40% of adults with diabetes will develop chronic kidney disease, a population Renalytix is focused on through its kidneyintelX.dkd prognostic test.1 By helping clinicians identify which patients are most at risk for progression, the goal is to enable earlier, more targeted intervention.
As a pathologist with a background in oncology and translational medicine, Dr. Donovan has spent his career studying how biomarkers can predict disease risk and guide treatment. Now, he’s applying those lessons to kidney disease, where predictive tools could help millions of people avoid kidney failure altogether.
His key takeaway?
“When you combine biomarker biology with clinical data, you elevate precision medicine from population statistics to individual insight.”

What’s Inside:
How Dr. Donovan’s background in oncology shaped his approach to kidney disease
Why combining biomarkers with clinical data is transforming early detection
The role of machine learning in creating personalized risk scores
What real-world evidence reveals about physician behavior and patient outcomes
How predictive medicine could improve both costs and quality of care
Q&A
You’ve had a long career in translational medicine and pathology. What drew you to kidney disease?
MD: I’m a pathologist by training. I completed my residency at New York Hospital–Cornell, then a fellowship in pediatric pathology at Boston Children’s Hospital. My early research focused on developmental biology and gene discovery. Eventually, I joined Millennium Pharmaceuticals, where I helped lead their translational research program focused on predictive medicine in oncology.
Over the years, I became fascinated with biomarkers, these measurable biological signals that can tell us what’s really happening in the body and predict how disease will progress. Most of my work had been in cancer, but when I connected with Renalytix’s leadership and saw their approach to kidney disease, it immediately resonated. Here was a chronic disease affecting hundreds of millions worldwide, yet still being managed reactively. The idea of applying personalized, data-driven tools to kidney care was both new and deeply familiar to me.
At Renalytix, I now serve as Chief Medical Officer and as the medical director of our laboratory in New York. Because New York has unique requirements for laboratory oversight, I’m also licensed to sign all our tests—a rare intersection of clinical medicine, lab operations, and translational science.
You mentioned coming from oncology. What parallels did you see between cancer and kidney disease?
MD: In oncology, especially in the early 2000s, we were obsessed with predictive medicine. We were a bit naïve. We thought, “If we just sequence enough genes, we’ll find the magic bullet.” But biology isn’t that simple. Disease is complex, and we learned that understanding its progression required looking at multiple systems at once.
That lesson translates directly to kidney disease. Much like cancer, CKD is driven by immune and cellular processes that can vary from person to person. What’s striking is how much of it is immunologically mediated. The same inflammatory pathways play a major role in both cancer and chronic kidney disease. Once you realize that, the parallels become clear.
So the work we do now, measuring immune-driven biomarkers in the blood and using them to predict disease progression, is a natural extension of the same principles we used in oncology. It is personalized medicine applied to a chronic condition that, until recently, had almost no predictive tools.
How do you think about the scale of kidney disease, and where to intervene?
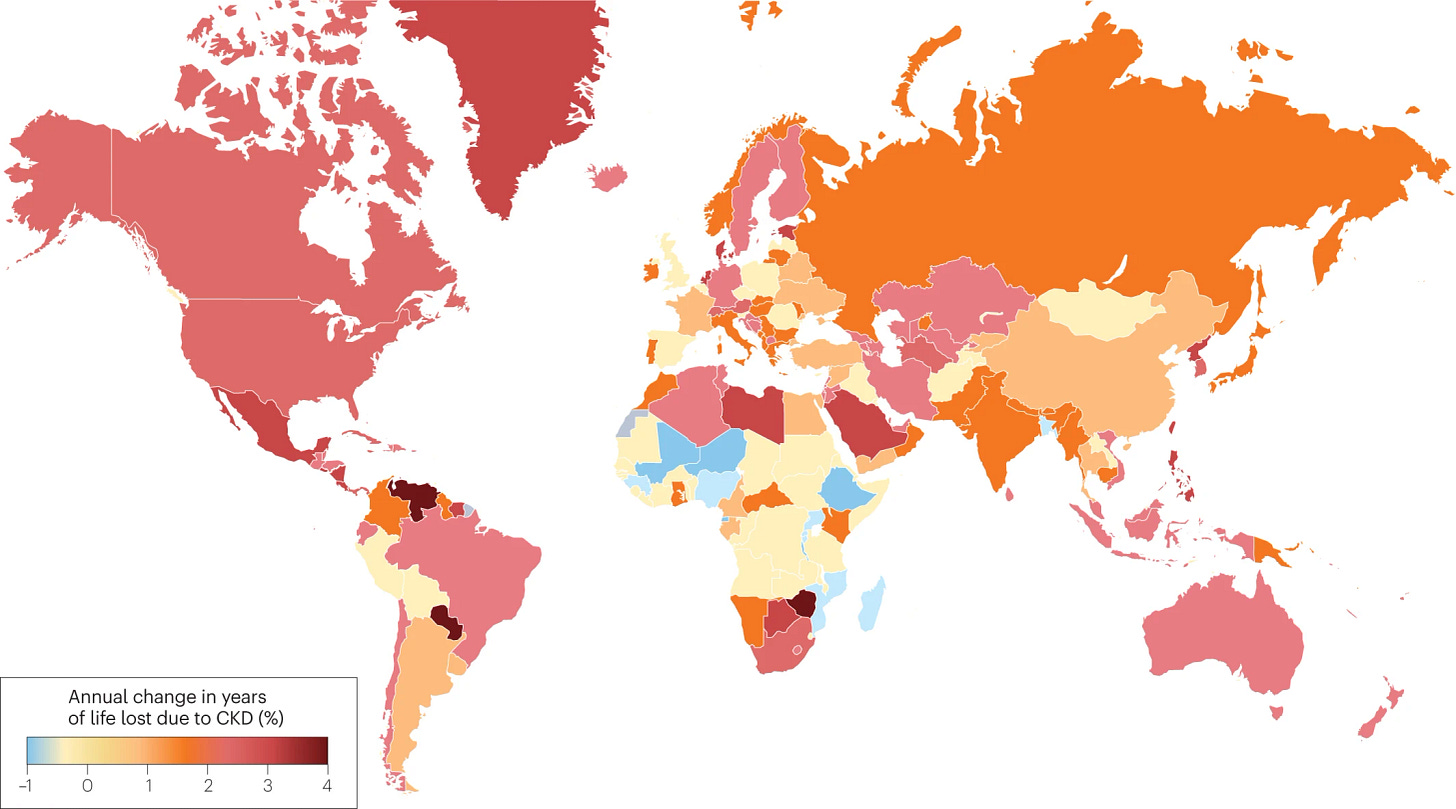
MD: When I first stepped into this field, the scale shocked me. Over 840 million people globally are living with chronic kidney disease, and in the U.S. alone, around 37 million. Among them, 14–15 million have early-stage diabetic kidney disease. That’s an enormous population.2
Figure: Predicted change in years of life lost to chronic kidney disease from 1990 to 2040.3
If you want to make an impact, you must move upstream—to the early stages of disease. Once kidneys fail, the options are limited: dialysis or transplant. But if you can identify who’s at risk early and intervene, you can change the entire trajectory. That’s where we focus: patients with Type 2 diabetes and early-stage CKD, before irreversible damage occurs.
Our approach combines two powerful data sets: traditional clinical variables (like blood urea nitrogen, estimated glomerular filtration rate, and hemoglobin A1C) and biomarker biology—proteins in the blood that reflect underlying inflammation and immune activity. Together, they create a much clearer picture of who’s likely to progress and who isn’t.
How does machine learning enhance that picture?
MD: Historically, physicians relied on population-based metrics like estimated glomerular filtration rate (eGFR) or urine albumin-to-creatinine ratio (UACR). Those are useful, but they only tell part of the story. You could have a perfectly normal eGFR and still have early disease that’s silently progressing. Both tests are needed to diagnose diabetic kidney disease (DKD), but each comes with its own variability—results can differ significantly from test to test depending on hydration, lab timing, and other factors. Together, they offer a snapshot of current kidney function and damage, not a view into who is most likely to experience future progression to kidney failure or require dialysis or transplant.
Machine learning lets us look at patterns that traditional statistics can’t capture. Instead of analyzing one or two variables, we can evaluate dozens of clinical and biomarker features simultaneously; how they interact, amplify, or offset each other. In practice, this means taking a patient’s blood, measuring their biomarkers, and combining those results with their clinical data through a trained algorithm. The output is a personalized risk score: low, moderate, or high.
Figure: kidneyintelX.dkd Validation Study4
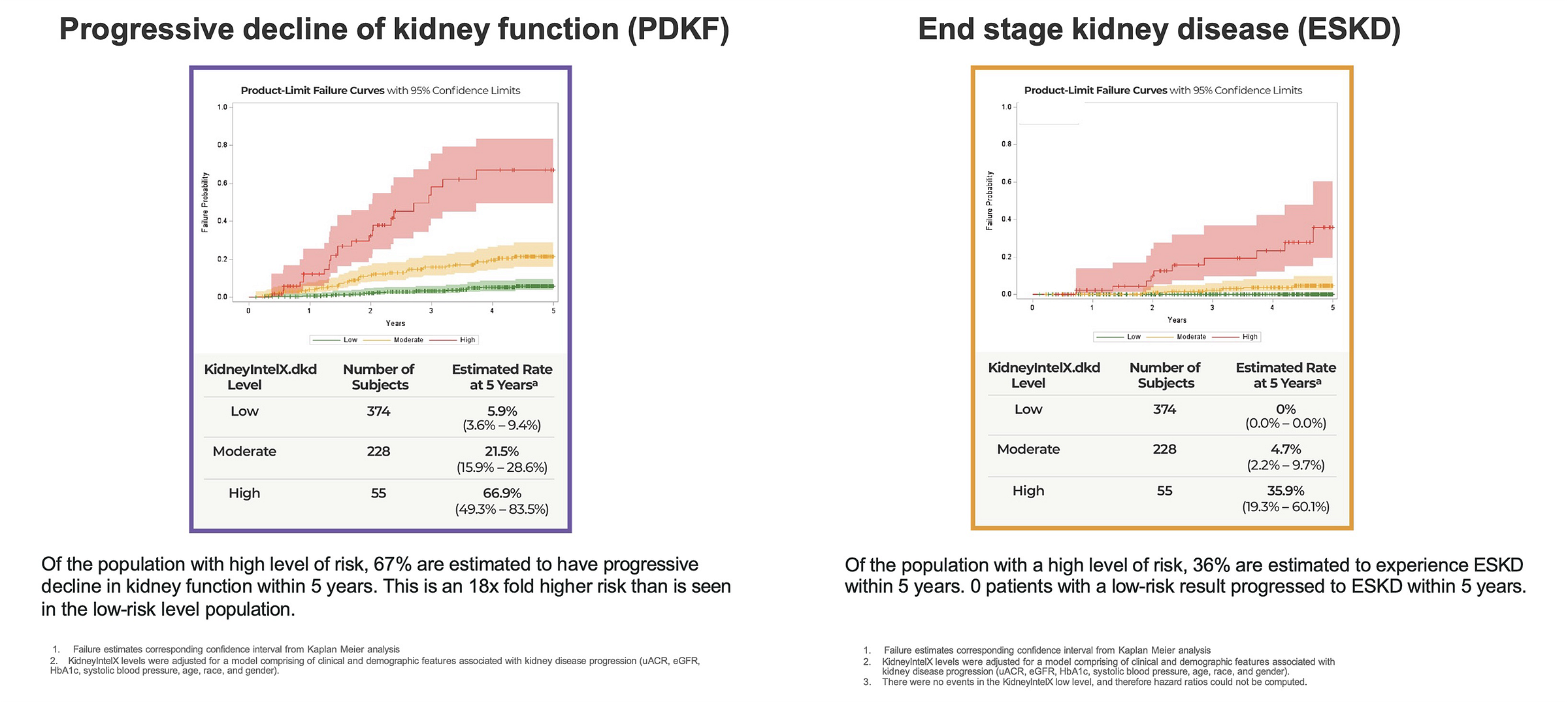
That risk score isn’t abstract—it correlates with real outcomes. In the clinical validation study, kidneyintelX accurately assessed the risk of progressive decline in kidney function. Patients identified as “high” risk experienced kidney function decline at a ratio of approximately 18x greater than those with a “low” result. Those in the “moderate” group declined at about 4x greater than low-risk patients. Low-risk patients experienced kidney function decline comparable to that of normal aging (in the absence of diabetes or CKD), and zero progressed to end-stage kidney disease within five years.5
So when a clinician orders the test, what do they actually see? How do they use it?
MD: Our test, kidneyintelX.dkd, produces a simple and intuitive risk result report: low, moderate, or high risk. That risk level corresponds to the likelihood of a significant decline in kidney function (defined as a sustained 40% drop in eGFR) or progression to kidney failure.
Physicians can align that risk level with existing clinical guidelines. For low-risk patients, the message is “stay the course”—maintain current care, encourage medication adherence, and monitor as usual. Moderate-risk patients require closer follow-up and perhaps medication adjustments and lifestyle modifications. High-risk patients are where you act aggressively—consider referring to nephrology, intensifying therapy, including layering of multiple protective drugs like SGLT2 inhibitors and GLP-1s that benefit both kidney and heart health.
The beauty of it is simplicity. Busy clinicians can quickly interpret the results and integrate them into care planning. It’s not adding complexity—it’s adding clarity.
What have you learned from the real-world use of the test so far?
MD: Quite a lot. At Mount Sinai, we ran a real-world evidence study with over 10,000 patients to see how risk information changed behavior. The results were encouraging.
High-risk patients were seen more frequently, often through telemedicine during the pandemic. Physicians referred them more often to nutrition, endocrinology, and nephrology. Moderate-risk patients also received more proactive management. And we saw measurable physiologic improvements: better blood pressure control, lower A1C levels, and even improved kidney function slopes over one year.
Figure: New SGLT2i prescriptions post-KidneyIntelX result stratified by risk and referring physician.
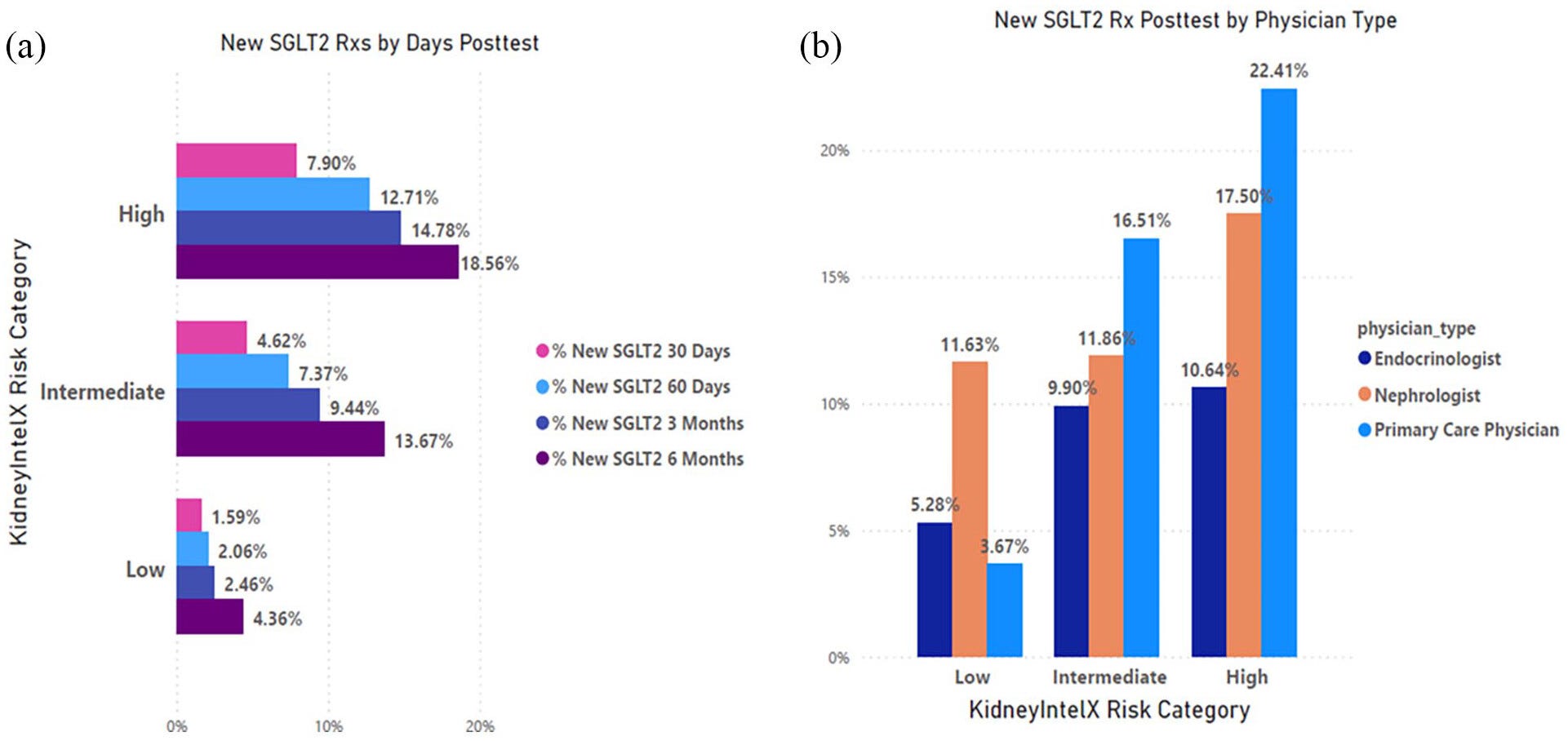
Perhaps most striking was how these results translated into medication use. The adoption of SGLT2 inhibitors (drugs that protect both the kidney and heart) rose dramatically among patients classified as moderate or high risk. Physicians were clearly using the information to guide evidence-based care.
That’s the power of predictive data. It turns education into action.
How does this complement traditional staging tools like the kidney “heat map”?
Clinicians often use a tool called the KDIGO Heat Map—a chart that cross-references eGFR and UACR levels to stage kidney disease from G1 to G5 (see below).6 It’s useful, but limited. Many primary care physicians only look at eGFR, so if that number looks fine, they assume the patient’s kidneys are healthy.
Figure: KDIGO Heat Map
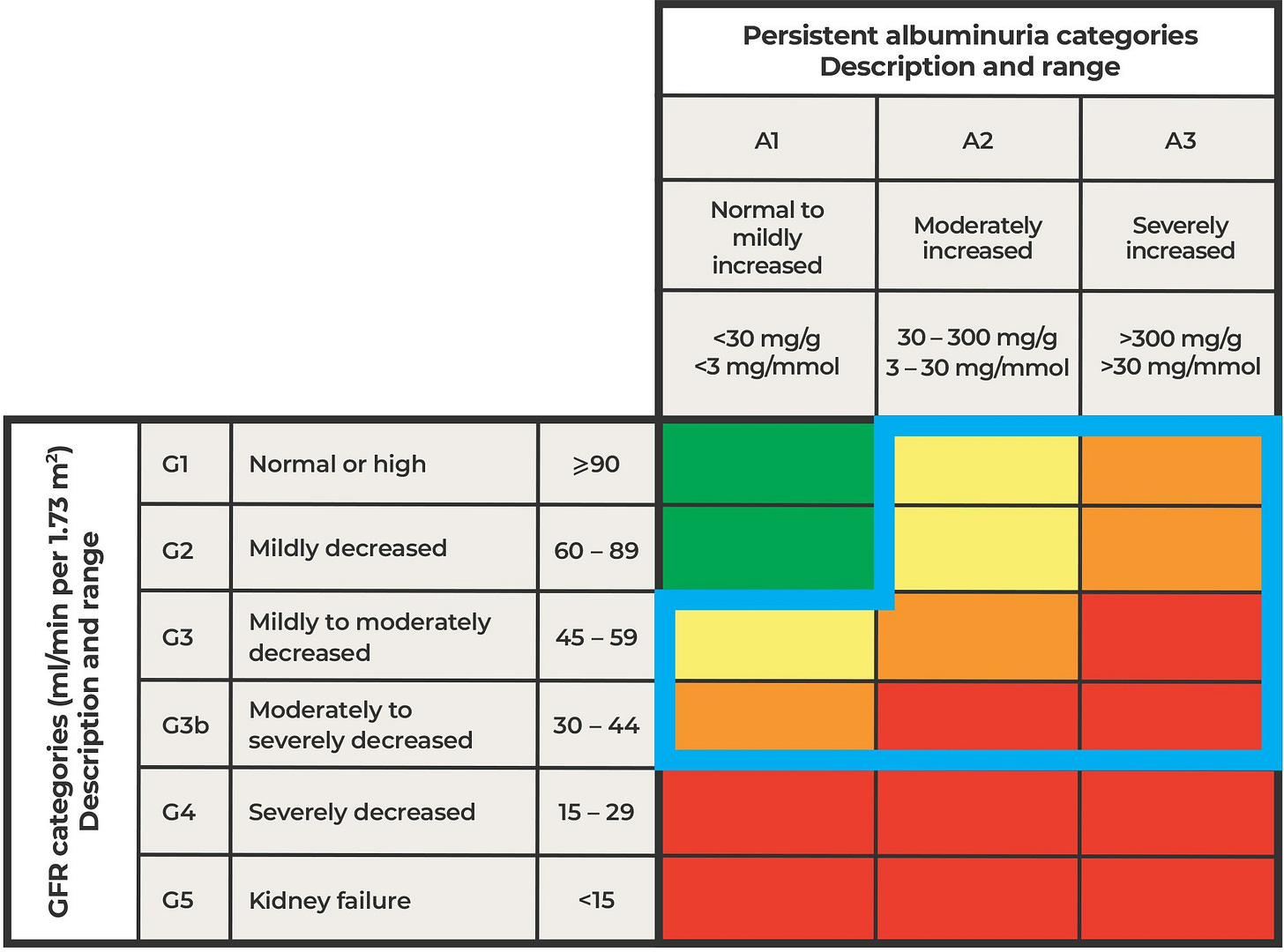
What we found in our real-world studies is that some patients classified as low stage on that map—say, G1 or G2—were actually high risk based on our test. Their kidneys looked fine on paper, but the biomarkers told a different story. On the other hand, some patients in the G3 range turned out to be low risk, meaning their slower eGFR might just reflect normal aging rather than active disease.
So rather than replacing the heat map, our test enhances it. It helps physicians distinguish who truly needs stepped-up and more costly therapeutics and specialist care and who can safely be managed in primary care with the right medications and monitoring.
You mentioned the importance of primary care physicians. How are they responding?
MD: They’re central to this story. Most patients with early kidney disease never see a nephrologist—they’re managed by their primary care provider. So if we want to shift outcomes, that’s where we must start.
We spend a lot of time helping primary care practices identify eligible patients. In smaller offices, that can be as simple as using lab values to flag people with Type 2 diabetes and early CKD. In larger systems with electronic health records, we can automate the process and integrate the test directly into workflows.
Figure: Optimal care model by increasing severity of CKD
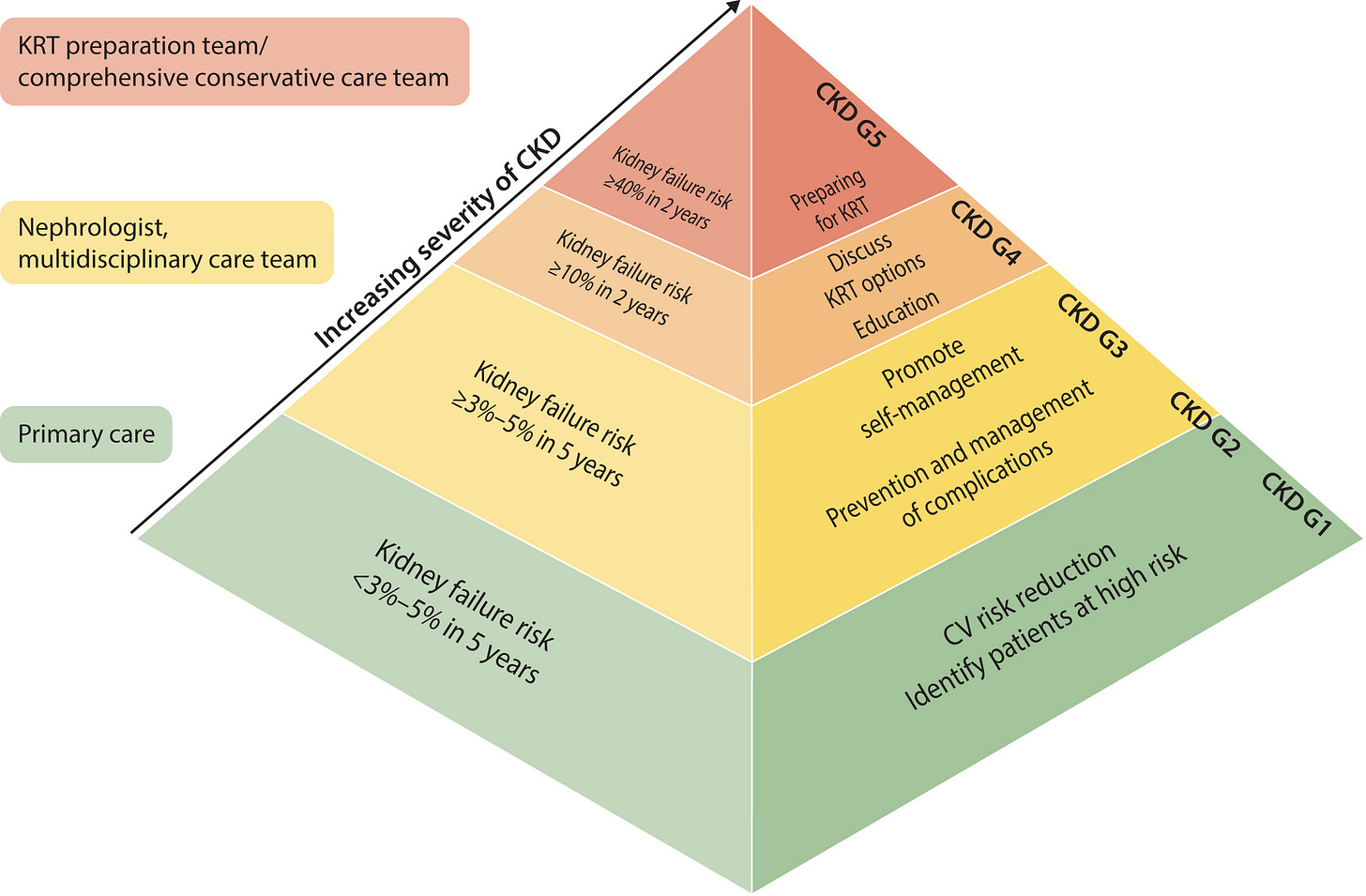
Once physicians see how straightforward it is, and that it’s FDA-approved and covered by Medicare (about 65% of our tested patients), the lightbulb goes off. It’s one more tool to prevent the scenario everyone dreads: a patient showing up in the emergency room for “crash dialysis,” completely unaware they had kidney disease.
How do patients respond when they see their results?
MD: It often changes the conversation. We had one case where a mother and daughter both had early CKD. The daughter’s test showed high risk, while her mother’s showed low risk. That opened a meaningful dialogue about diet, medication, and lifestyle. Without the test, they never would have known the difference—or taken action before it was too late.
It’s the kind of insight that helps patients feel informed rather than blindsided.
From a health system perspective, what’s the value case?
MD: The value is straightforward: prevention costs less than dialysis or transplantation. Kidney failure is one of Medicare’s largest expenditures ($1 of every $4 Medicare spends is on chronic kidney disease). If you can delay or prevent progression, you save not only lives but enormous resources.
Because our test is now FDA-authorized and Medicare-covered, health systems view it as de-risked—both clinically and economically. By accurately stratifying patients into low, moderate, and high risk, the kidneyintelX.dkd test can help clinicians focus advanced, high-cost therapeutics on the people who need them most.
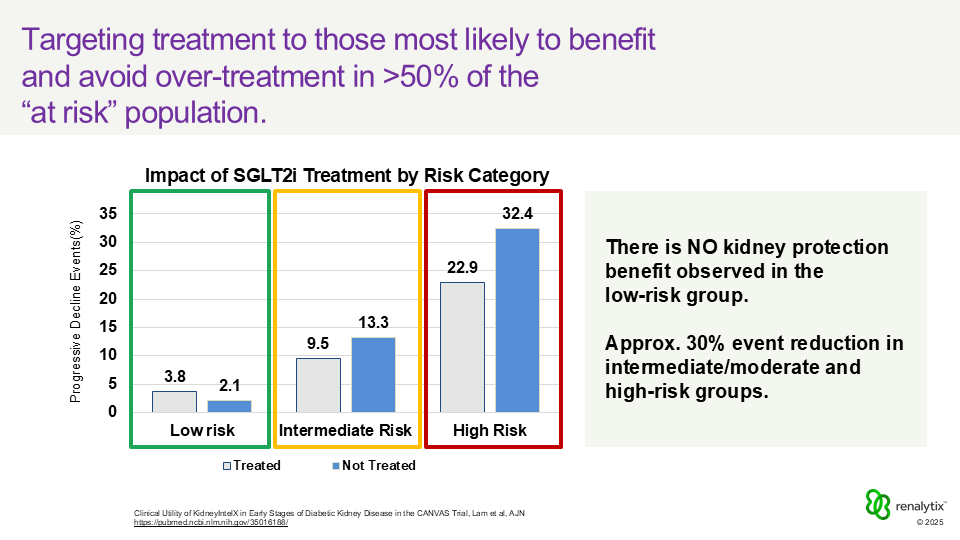
Figure: Impact of SGLT2i Treatment by Risk Category
Typically, about half of the patients tested fall into the low-risk category. That means their care often remains focused on guideline-based management and monitoring, while moderate and high-risk patients who are most likely to experience disease progression are prioritized for more aggressive therapy. In doing so, the test can streamline treatment decisions and cut unnecessary therapeutic spending roughly in half, which is meaningful for both Medicare and for patients’ out-of-pocket costs.
It’s value-based care in action: using precision tools to guide where interventions will make the greatest difference for patients, for clinicians, and for the healthcare system as a whole.
What’s top of mind for you over the next year?
MD: I’d say it’s two big things. First, expanding our real-world evidence programs to more health systems across the country. Mount Sinai was just the start. We want to include diverse populations across different geographies and care settings. That’s essential for proving the impact of predictive medicine at scale.
Second, advancing our research into companion diagnostics, linking specific biomarkers to drug response. The long-term vision is a world where kidney patients not only know their risk but can be matched to the therapy most likely to help them.
It’s a big challenge, but it’s where the field is headed. Personalized, preventive kidney care isn’t a distant goal anymore, it’s happening now.
Final thoughts for us?
The reality is simple: 90% of people with kidney disease don’t know they have it. That’s unacceptable in 2025.
If we can bring predictive tools like kidneyintelX.dkd into routine primary care, just like cholesterol or blood pressure screening, we can catch kidney disease progression early, treat it effectively, and prevent countless cases of kidney failure.
That’s the mission that drives us.
My thanks to Dr. Donovan for joining me to share his story and work. If you’re heading to ASN Kidney Week in Houston, you can meet the Renalytix team and see their latest research on Friday, November 7 (here, here, and here). For more on the kidneyintelX.dkd test, watch Michael’s video below or visit their website here.
Diabetes and Chronic Kidney Disease (National Kidney Foundation)
Renalytix Solutions (renalytix.com)
Francis, A., Harhay, M.N., Ong, A.C.M. et al. Chronic kidney disease and the global public health agenda: an international consensus. Nat Rev Nephrol 20, 473–485 (2024). https://doi.org/10.1038/s41581-024-00820-6
Nadkarni GN, Stapleton S, Takale D, et al. Derivation and independent validation of kidneyintelX.dkd: A prognostic test for the assessment of diabetic kidney disease progression. Diabetes Obes Metab. 2023;1‐9. doi:10. 1111/dom.15273
Chan, L., Nadkarni, G.N., Fleming, F. et al. Derivation and validation of a machine learning risk score using biomarker and electronic patient data to predict progression of diabetic kidney disease. Diabetologia 64, 1504–1515 (2021). https://doi.org/10.1007/s00125-021-05444-0
![Signals From [Space]](https://substackcdn.com/image/fetch/$s_!IXc-!,w_40,h_40,c_fill,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F9f7142a0-6602-495d-ab65-0e4c98cc67d4_450x450.png)
![Signals From [Space]](https://substackcdn.com/image/fetch/$s_!lBsj!,e_trim:10:white/e_trim:10:transparent/h_48,c_limit,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F0e0f61bc-e3f5-4f03-9c6e-5ca5da1fa095_1848x352.png)