When it comes to advancing kidney disease research, one of the biggest barriers is hiding in plain sight: finding the right patients for the right trials at the right time. For rare monogenic kidney diseases, the challenge is even steeper—small, geographically scattered patient populations, low rates of genetic testing, and a narrow window for enrollment.
Dr. Ronen Schneider, Medical Director of Renal Genetics at Natera, has spent his career at the intersection of nephrology, genetics, and precision medicine. Drawing on decades of research—including seminal studies on exomes and genetic testing—he’s helping translate the growing body of genetic knowledge into tools that work in everyday clinical practice. In this conversation, we discuss how his team is closing diagnostic gaps, accelerating clinical trial enrollment, and shaping the next era of kidney care.
What’s Inside:
Ronen’s path from clinical nephrology to genetic research
Why genetic testing is changing CKD diagnosis and management
The enrollment crisis in rare disease clinical trials—and how to fix it
Case study: 3× faster enrollment in an Alport syndrome trial
How Natera’s genetic counselors bridge patients, providers, and trials
Expanding the model beyond rare disease into CKD and other specialties
The human impact of precision enrollment strategies
Why nephrology is an emerging frontier for targeted therapies
This episode pairs nicely with my recent conversation with Bryce Powerman, a rare disease patient who walked us through the diagnostic and therapeutic odyssey from the patient’s side. Here, Dr. Schneider offers the clinician–researcher perspective—connecting the science, the operational hurdles, and the human stories behind a model that’s reshaping how patients find and access trials.
Q&A with Ronen Schneider
Tell us about your background and how you arrived at Natera.
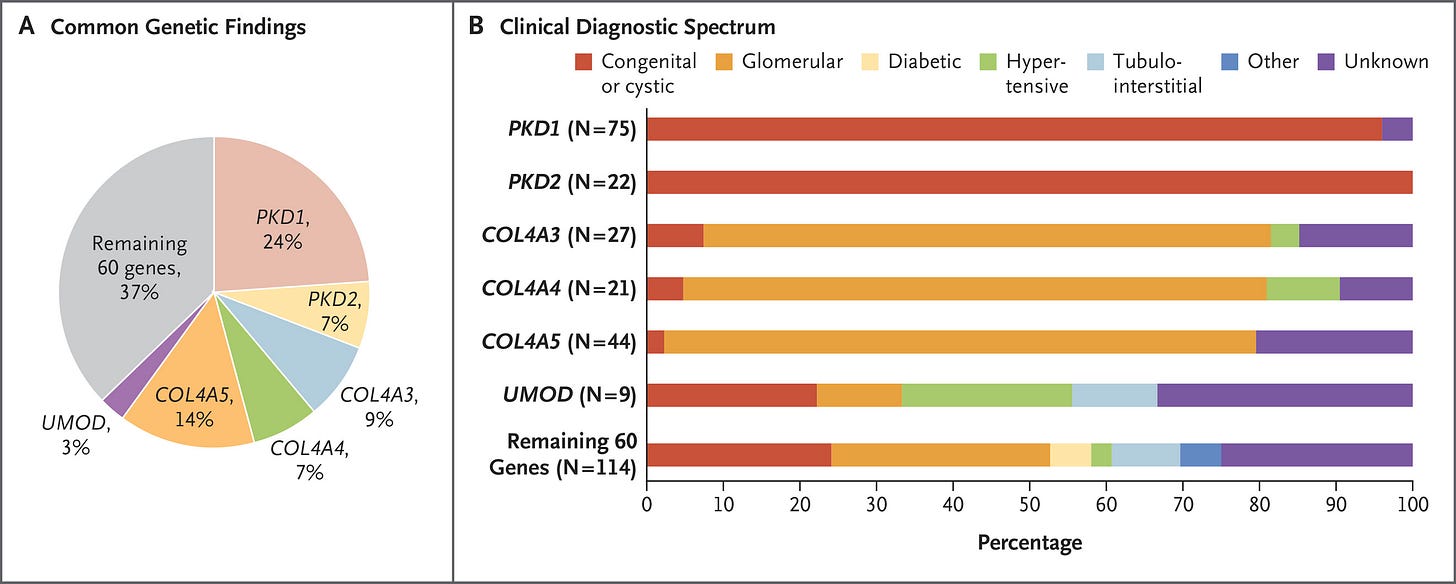
I recently joined Natera as Medical Director of Renal Genetics. I’m a nephrologist, trained at Hadassah Hebrew University Hospital in Jerusalem and later at Mass General Brigham in Boston. Early in my career, I saw the limitations of the “one-size-fits-all” approach in kidney disease management. That led me to join Dr. Friedhelm Hildebrandt’s lab at Boston Children’s Hospital, where I focused on gene discovery in pediatric CKD using whole exome sequencing.1
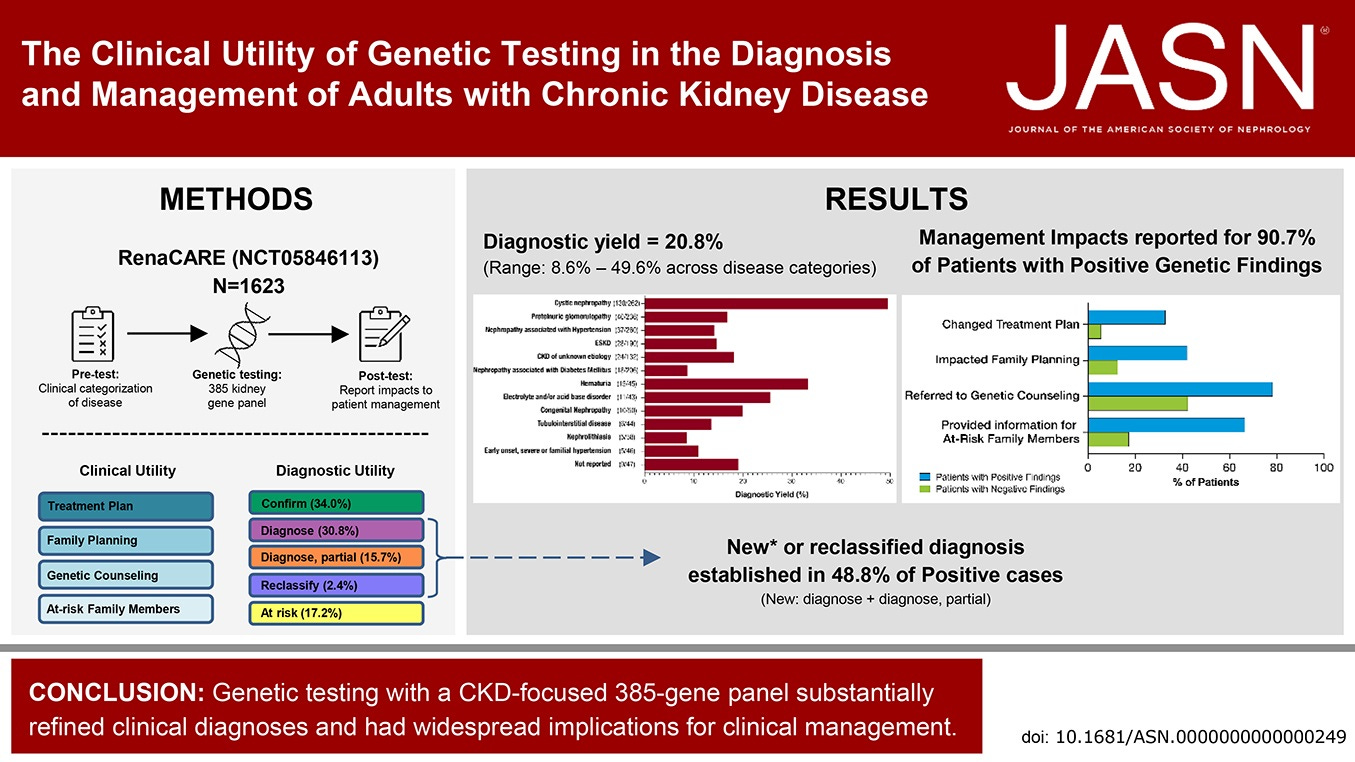
Over the past 2–3 decades, more than 600 genes have been identified as causative or associated with CKD. The burden of monogenic kidney disease can be as high as 40% in certain populations—not only in children, but also adults.2
With the introduction of Renasight™, renal care providers can now identify the genetic cause of CKD in ~20–25% of patients, and—more importantly—inform management decisions in over 90% of those with a positive result. Genetic diagnosis also connects patients to relevant clinical trials, including those for targeted therapies, bringing precision medicine closer to everyday practice.
From your clinical nephrology days to now, what’s the biggest change you’ve seen?
For decades we were in the gene discovery phase—learning which genes cause or contribute to kidney disease—but we didn’t have a practical tool clinicians could use. That changed about 4–5 years ago. Now testing is easy, widely available, and, for most patients, at no cost.
It’s a dramatic shift: a precise diagnosis in one in four or five patients. Many cases once labeled “unknown etiology” now have a name—and knowing the specific disease informs patients and guides treatment. Patients want answers, and they want to know how to treat their condition. This is a huge step toward true precision medicine.
Let’s talk about the clinical trial side. Why is patient enrollment such a challenge—especially in rare kidney diseases?
More than 80% of clinical trials fail to meet enrollment timelines, and ~30% fail altogether due to lack of participants. In rare diseases like monogenic CKD, the problem is even worse—about 55% of rare disease trials fail due to insufficient enrollment.
Challenges include:
Rarity of each subtype: While monogenic CKD accounts for up to 40% of CKD, each individual cause is rare and patients are geographically scattered.
Low genetic testing rates: Many eligible patients are never tested due to lack of provider awareness.
Diagnostic delays: Patients often present too late to qualify for early-intervention trials.
Limited longitudinal data: Historically, outcomes and disease progression were poorly documented.
Few robust registries: This makes pre-screening and identification of patients harder.
Natera’s centralized genetic and clinical database directly addresses these gaps.
How Testing Works
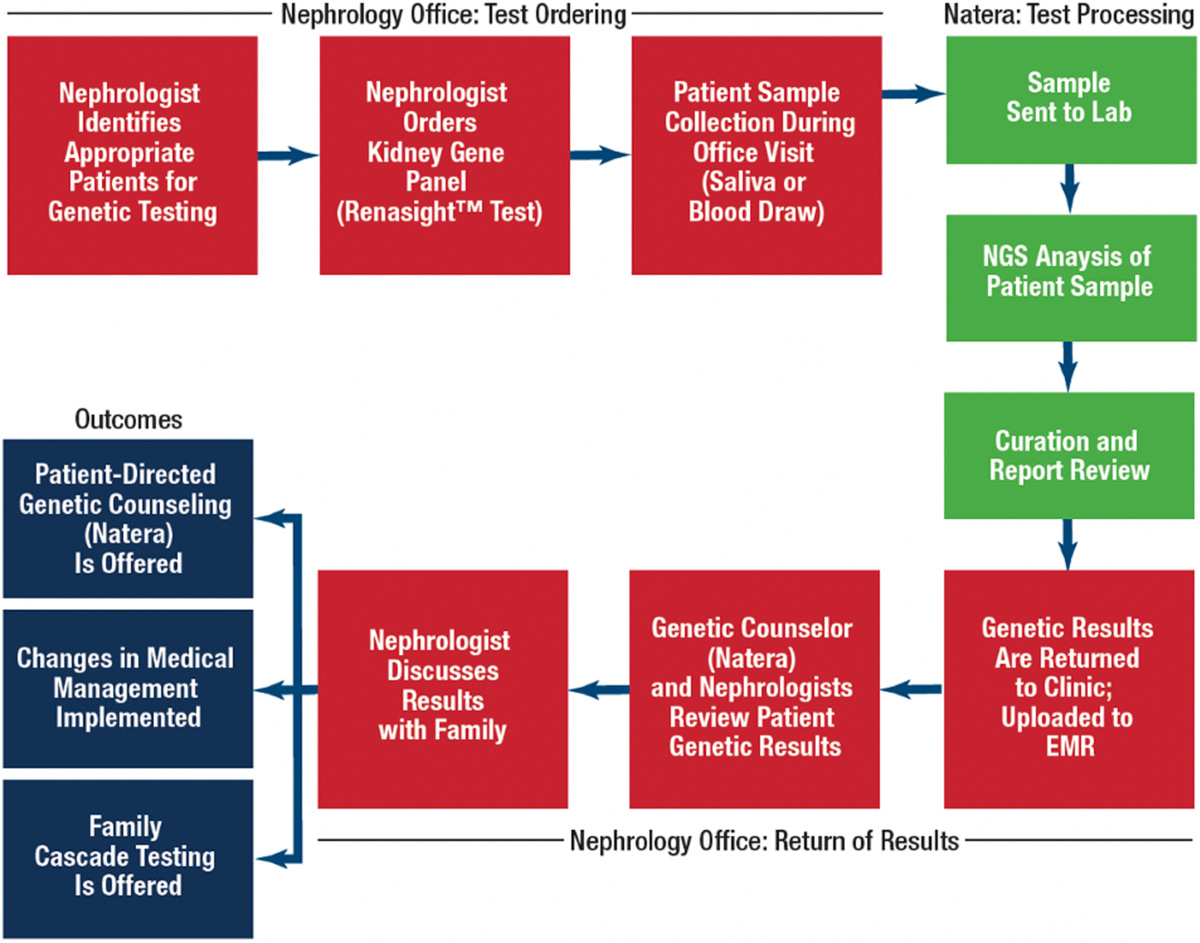
Can you walk us through how your centralized database works in practice?
Sure—let me use the ENYO Pharma case study as an example. ENYO was running a Phase 2 trial for Vonafexor in X-linked recessive Alport syndrome (XLAS, affects ~1 in 5,000 people). They partnered with Natera’s RenasightIQ™ clinicogenomic database to identify eligible patients.
Using our genetic database of 170,000+ patients (enriched with clinical and claims data), we: (a) applied sponsor-defined inclusion/exclusion criteria; (b) selected optimal trial sites based on patient geography; and (c) used our genetic counselor team to pre-screen and directly engage patients and referring physicians.
As a result, ENYO reached 100% of its enrollment target in 6 months, compared to the industry average of 16 months—a 2.5× faster enrollment and 10-month timeline reduction, with significant cost savings.3
How does your team actually work with physicians and patients to close the loop?
We don’t just identify patients—we engage them. Our genetic counselors reach out to referring physicians and, with consent, directly to patients to guide them through trial information and enrollment. This human connection builds trust and accelerates participation.
Our genetic counselor team is available before and after testing—for any patient or provider using Renasight—and also leads outreach for relevant clinical trials. Patients can opt in or out of being contacted about trials.
Can you share a bit more on the human impact?
Absolutely. In Alport syndrome, there’s no curative treatment—most males with the X-linked form require kidney replacement therapy by early adulthood. Trials like ENYO’s offer a chance to change that trajectory.
Another example: a young woman with severe proteinuria and kidney failure had a biopsy showing FSGS. Traditionally, we would treat with steroids, but her Renasight results revealed an APOL1 high-risk genotype. This allowed us to avoid ineffective steroid therapy and instead refer her to a trial for Enoxaparin, a promising molecule in APOL1-related FSGS.4
Beyond rare disease, where else can this model be applied?
The approach—integrating genetic and clinical data to optimize trial design and recruitment—applies to common CKD forms like diabetic nephropathy, IgA nephropathy, and hypertensive nephrosclerosis.
As biomarkers and genetic stratifiers become validated, precision enrollment will be essential. We can reduce screen failure rates and increase statistical power by creating smaller, better-defined cohorts.5
Beyond nephrology, the model can be applied to cardiology, rare autoimmune or metabolic diseases, and oncology. Truly any field where patient heterogeneity complicates trial design.
Since joining Natera, what’s been the biggest surprise for you?
I knew Renasight was impactful, but seeing the behind-the-scenes numbers has been eye-opening. Drug development in nephrology is moving from a one-size-fits-all model to one driven by genetics, biomarkers, and personalized data.
Genetic platforms like RenasightIQ are not just diagnostic tools—they’re accelerators for drug development, patient matching, and better trial design. For patients, it means awareness of trials they never would have known about before.
Looking ahead, what’s the opportunity?
Nephrology has been overlooked for too long. Now, with genomic and clinical data capabilities, it’s becoming an emerging frontier for targeted therapies. We should embrace this momentum and foster collaboration across diagnostics, pharma, and data science to bring the next generation of treatments to patients faster.
My thanks to Dr. Schneider for joining us and sharing his story. For more on the power of genetic testing in kidney disease, see the 2023 JASN RenaCARE study, where 1 in 5 patients tested positive for a genetic cause of CKD and 1 in 3 had a change in treatment. A 2024 collaboration with the Alport Syndrome Foundation highlighted common COL4 gene variants and the evolving role of precision diagnostics in rare kidney disease. In 2025, a study published in Urolithiasis used Renasight to identify genetic drivers in high-risk kidney stone patients, reinforcing the value of testing in recurrent stone disease. And back in 2022, the Renasight 1000 study revealed that 25% of early patients tested had a genetic finding, with nearly half carrying rare variants not detectable with limited panels. For more on genetic kidney diseases, read this recent special section of Kidney News with expert views and patient perspectives on Fabry disease, Alport syndrome, and more.
KDIGO Conference Participants. Genetics in chronic kidney disease: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Kidney Int. 2022 Jun;101(6):1126-1141. doi: 10.1016/j.kint.2022.03.019. Epub 2022 Apr 20. PMID: 35460632; PMCID: PMC9922534.
Groopman EE, Marasa M, Cameron-Christie S, Petrovski S, Aggarwal VS, Milo-Rasouly H, Li Y, et al. Diagnostic Utility of Exome Sequencing for Kidney Disease. N Engl J Med. 2019;380(2):142-151. doi:10.1056/NEJMoa1806891.
Data on file. Natera, 2024.
Lin R, McDonald G, Jolly T, Batten A, Chacko B. A Systematic Review of Prophylactic Anticoagulation in Nephrotic Syndrome. Kidney Int Rep. 2019 Dec 12;5(4):435-447. doi: 10.1016/j.ekir.2019.12.001. PMID: 32274450; PMCID: PMC7136344.
In clinical trials, statistical power refers to the probability that a study will correctly detect a real effect or difference, if one truly exists in the population. It essentially measures the ability of a study to avoid a false negative conclusion (Type II error). A statistically powerful study has a high chance of finding a significant result when there is a real effect, while an underpowered study might fail to detect a real effect, leading to a false negative.
![Signals From [Space]](https://substackcdn.com/image/fetch/$s_!IXc-!,w_40,h_40,c_fill,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F9f7142a0-6602-495d-ab65-0e4c98cc67d4_450x450.png)
![Signals From [Space]](https://substackcdn.com/image/fetch/$s_!lBsj!,e_trim:10:white/e_trim:10:transparent/h_48,c_limit,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F0e0f61bc-e3f5-4f03-9c6e-5ca5da1fa095_1848x352.png)
![Signals From [Space]](https://substackcdn.com/image/fetch/$s_!NnOt!,w_144,h_144,c_fill,f_auto,q_auto:good,fl_progressive:steep,g_auto/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F688fc47b-7202-4a2e-b4f4-fea2b047ab1b_1500x1500.png)