July was another busy month for kidney health, from the World Transplant Congress to a wave of industry and regulatory developments. CMS rolled out its proposed 2026 ESRD PPS rule, reshaping quality and payment models in areas like patient experience surveys, health equity reporting, and base reimbursement rates for both in-center and home dialysis. On the regulatory front, the FDA granted a notable new approval that could expand therapeutic options for kidney patients, while new joint ventures and acquisitions signaled continued investment momentum in the space. We’re also seeing growing questions about funding—both public and private—against a backdrop of potential Medicaid cuts that could impact access and innovation. Comments on the proposed fee schedule are due by September 12, and I’m looking forward to seeing what the next 30 days bring in shaping global kidney health. Thank you for being here with us.1
Have tips or feedback? Hit reply or drop us a note here.
Reading time: 25 minutes
What’s Inside
News: Vexev & US Renal Care complete enrollment in AI-assisted AVF mapping trial, Frenova partners with Nephronomics & GENEWIZ on 35K-patient genomics registry, CorMedix to acquire Melinta in $300M acute care expansion, Linea & DaVita report 50% heart failure readmission drop with AI platform, Xeltis posts positive EU data for CABG conduit, FDA begins review of iBox transplant trial endpoint and approves first therapy for C3G & IC-MPGN, Vinay Prasad reinstated…
Research: AST survey finds heavy side effect burden from transplant immunosuppression, SRTR data show DCD donors surpass DBD for first time, genomic biobanks advance CKD genetics insights, new ADPKD genetic testing guidelines, large study links physician affiliation to site-of-care cost gaps, ARPA-H project develops autonomous robotic kidney surgery…
Community: Singapore GH pioneers Therasorb therapy and showcases research at WTC, Fresenius hosts Asia Pacific home therapies summit, NephCure’s SEISMIC summit sets rare kidney disease priorities, NCQA convenes CKD quality panel, CHAI supports CMS direct-to-patient health data tools, Signals shares 25 kidney transplant questions at WTC…
Events: Budapest Nephrology School, SLANH 2025 in Guayaquil, Russian Dialysis Society meeting in Moscow, OSNT in Oman, LSI Emerging MedTech in London, Women in Medicine Summit in Chicago, ASN Kidney Week in Houston…
Jobs: Kaüna, Vertex, Edwards, Monogram, Sanofi, US Heart and Vascular, AstraZeneca, Vantive, Travere, CareDx, Cityblock, Ardelyx, Mendaera, Intermountain, and more…
This issue is made possible by Natera. Natera supports personalized kidney care and drug development through advanced genetic testing and insights. RenasightIQ™ is a robust clinicogenomic database of 170,000+ chronic kidney disease patients enriched with longitudinal clinical and claims data. This powerful platform delivers real-world insights from early investigation to commercialization to accelerate the kidney disease drug development lifecycle.
News
INDUSTRY
CANSCAN trial completes enrollment for AI-assisted AVF mapping in dialysis care. Vexev and US Renal Care’s 120-patient study is evaluating the VxWave semi-autonomous ultrasound system, designed to perform vascular mapping for dialysis arteriovenous fistulas directly in the clinic. By combining robotics, machine learning, and advanced ultrasound processing, the system could streamline access creation, reduce extra imaging visits, and speed placement of permanent vascular access for patients with kidney disease (MassDevice).
Mendaera scores FDA clearance for handheld robotic needle delivery system. The Focalist system clips onto a handheld ultrasound probe to guide precision needle placement for procedures such as central line insertions, kidney stone removal, and biopsies. Early studies showed higher first-stick accuracy across all skill levels, with novices outperforming experienced specialists. Mendaera plans a limited commercial launch this year in urology before expanding to other specialties in 2026, with future AI and telehealth capabilities in development (Fierce Bio).

Frenova, Nephronomics, and GENEWIZ team up on genomics-driven kidney care. The partnership will sequence and analyze biospecimens from over 35,000 participants in Frenova’s My Reason® registry, aiming to reach 50,000 in two years. By combining large-scale genomic data with AI and machine learning, the initiative seeks to uncover disease subtypes, protective variants, and new therapeutic targets—advancing precision medicine for cardio-kidney-metabolic diseases worldwide (Fresenius Medical Care).
CorMedix to acquire Melinta Therapeutics, expanding acute care portfolio. The $300M deal will add seven marketed products and boost CorMedix’s presence in hospital and clinic settings. Projected to generate $305–$335M in 2025 revenue with $35–$45M in annual synergies, the acquisition also brings pipeline growth potential, including a BARDA-backed pediatric development for key antibiotics (CorMedix).
Biogen launches ‘New Ventures’ to externalize early R&D. Following research unit cuts, the Boston biotech will back external startups and assets, allowing innovation to develop outside its labs before potential in-licensing (Endpoints News, h/t Adam Keeney, PhD).
Linea expands AI-powered platform to cut heart failure readmissions by 50%. Backed by Redesign Health and DaVita, Linea’s 90-day post-acute care program uses AI-driven admission detection, prescribing optimization, and real-time engagement to reduce readmissions, improve outcomes, and lower costs for ACOs. The platform also targets the high-risk overlap of heart failure and kidney disease, achieving sub-25% readmission rates and engaging most patients before hospital discharge (Redesign Health).
Xeltis reports positive EU trial data for Xabg coronary bypass conduit. In a single-arm feasibility study, the bio-restorative, polymer-based graft showed promising safety, patency, and blood flow in multi-vessel coronary artery disease patients undergoing elective CABG surgery. Xabg is Xeltis’ second product in development after aXess, its restorative vascular access conduit now in pivotal EU and US trials for dialysis patients—highlighting the company’s growing cardiovascular and kidney-focused pipeline (Xeltis).
Balboa Research joins Thermo Fisher’s PPD Select Partnership Program. As a preferred site within PPD’s Strategic Site Collaborations network, Balboa will expand its clinical research capabilities, strengthen patient access to novel therapies, and create new opportunities for advanced, high-impact studies (LinkedIn).
Strive Health adds CTO and Chief Actuary — Tom Hawkes joins as Chief Technology Officer, bringing nearly 20 years of healthcare technology and product leadership. Amit Trivedi joins as Chief Actuary with three decades of actuarial and medical economics expertise. Both will focus on advancing technology, risk management, and value-based care strategies to improve outcomes and drive sustainable growth (Strive).
POLICY / REGULATORY
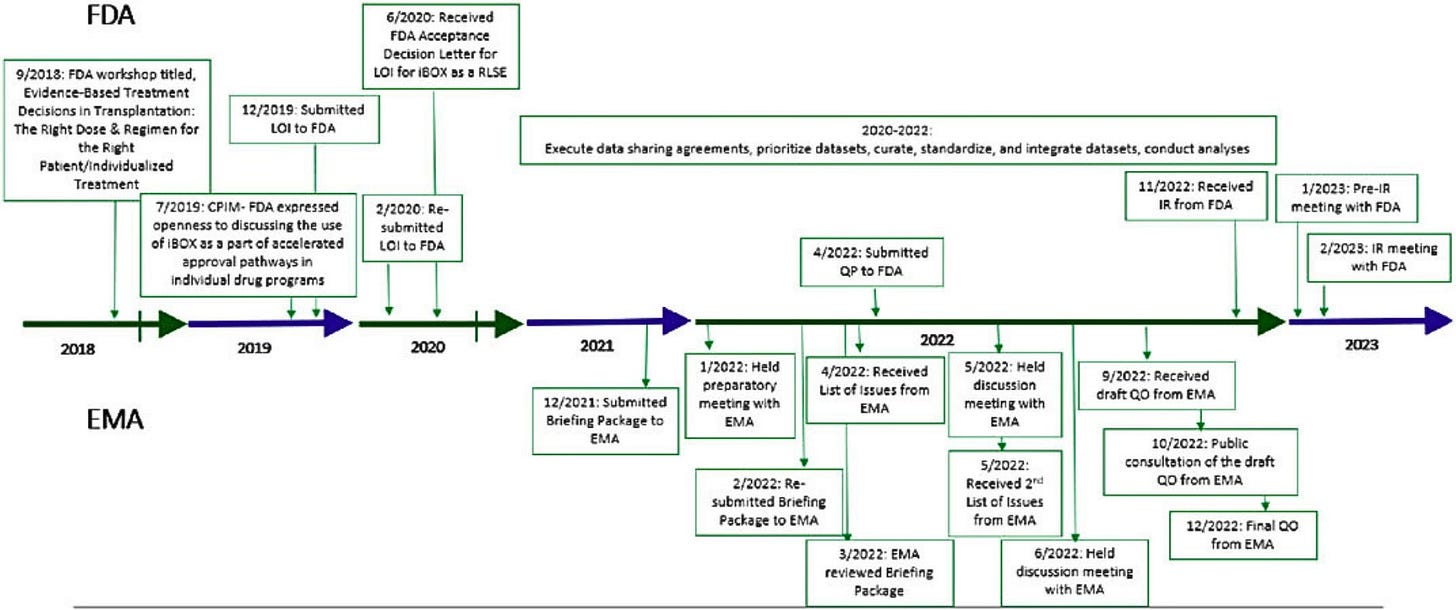
FDA begins review of iBox Scoring System for transplant drug trials. The Transplant Therapeutics Consortium’s composite biomarker, which predicts 5-year kidney graft survival from one-year post-transplant data, is the first transplant endpoint to reach the Full Qualification Package stage. If approved, iBox could serve as the first qualified “reasonably likely” surrogate endpoint in transplantation, enabling use in the FDA’s accelerated approval pathway for new immunosuppressants (Transplant Therapeutics Consortium, h/t Karin Hehenberger).
FDA approves EMPAVELI® as first treatment for C3G and primary IC-MPGN. Apellis’ pegcetacoplan is now cleared for patients 12 and older, including post-transplant recurrence, after the Phase 3 VALIANT trial showed a 68% proteinuria reduction, kidney function stabilization, and C3 deposit clearance in most patients. The approval marks a major advance for two rare kidney diseases that often progress to kidney failure within a decade and previously had no approved therapies (Apellis).
FDA declares US shortage of IV and injectable saline resolved. Supply disruptions caused by Hurricane Helene’s damage to Baxter’s North Carolina plant—responsible for 60% of national IV fluid and peritoneal dialysis solution output—had forced hospitals to delay elective procedures in 2024. Production ramp-ups by Fresenius and B. Braun, along with Baxter’s imports and restoration of inventory, have ended the shortage, though the FDA will continue monitoring other IV fluid supplies still in deficit (Reuters).
Vinay Prasad reinstated as head of FDA biologics center. Just two weeks after his departure, Prasad will resume leading the Center for Biologics Evaluation and Research, which oversees vaccine, gene therapy, and blood product regulation. His return follows tensions over Sarepta’s Duchenne muscular dystrophy therapy and political controversy; HHS Secretary Robert F. Kennedy Jr. and FDA Commissioner Marty Makary had opposed his dismissal (STAT, Politico, Reuters).
CMS proposes expanded Medicare coverage for remote patient monitoring without new guardrails. The 2026 physician fee schedule would allow billing with less data collection and patient interaction, aiming to stabilize payments and encourage broader use of RPM for chronic and acute conditions. While vendors welcome the changes, watchdogs warn that the program—whose Medicare spending grew from $15M in 2019 to over $300M in 2022—still lacks safeguards to prevent overuse and ensure it targets patients most likely to benefit (STAT News).
Research
MARKETS
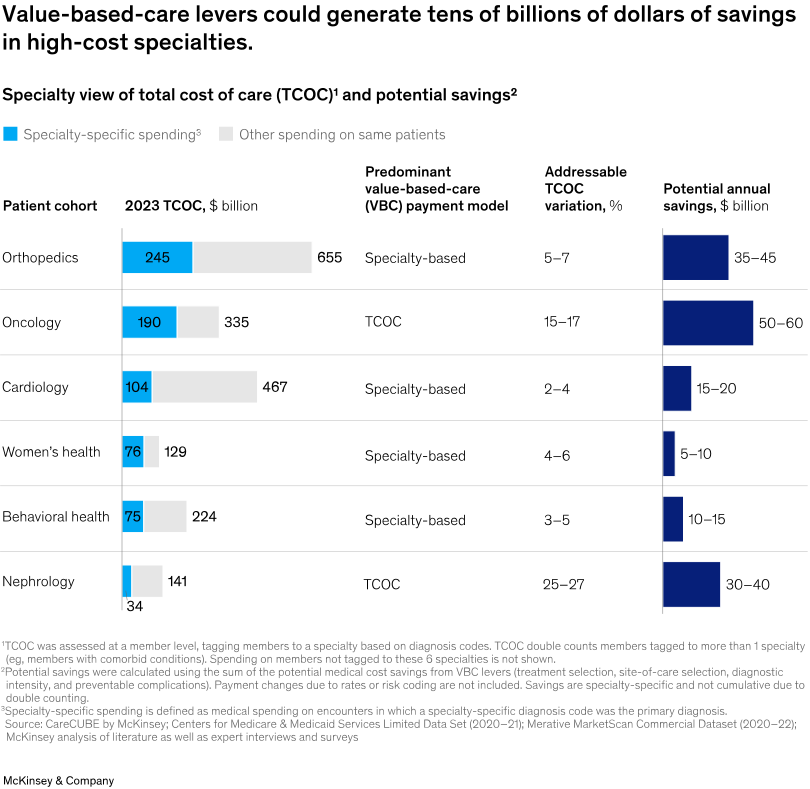
Value-based specialty care could save US healthcare $100B annually. A new McKinsey report finds that specialty services like nephrology, cardiology, and oncology now account for over 40% of total medical spending and drive most cost growth (see below), yet remain underrepresented in risk-bearing value-based care contracts. The analysis outlines four strategies—wraparound services, outpatient-integrated care, treatment pathway navigation, and digital health innovation—to address specialty care spending through better diagnostics, therapy selection, site-of-care shifts, and complication prevention (McKinsey & Co).
HSBC reports strong Q1 but slower Q2 in 2025 healthcare venture funding. Mega rounds of $150M+ drove the biggest investment quarter in two years, with 20 large deals—12 in biopharma and 6 in healthtech—making up a third of Q1 dollars. However, economic, geopolitical, and industry uncertainty slowed Q2 activity, with invested capital down 20% from Q1. Many companies still on insider rounds struggled to find new lead investors, often relying on smaller insider extensions paired with aggressive cost-cutting to extend runway (HSBC).
Study highlights site-of-care cost gaps tied to physician affiliation. An analysis of high-volume specialty procedures found hospital-affiliated physicians were least likely—and private equity–affiliated physicians most likely—to use lower-cost settings, with procedure prices consistently higher in hospital-based care under both Medicare and commercial plans. The findings point to site-of-care strategy as a lever for independent practices to deliver higher-value care and to policy options like site-neutral payments to curb spending growth (h/t Dan O’Neill).
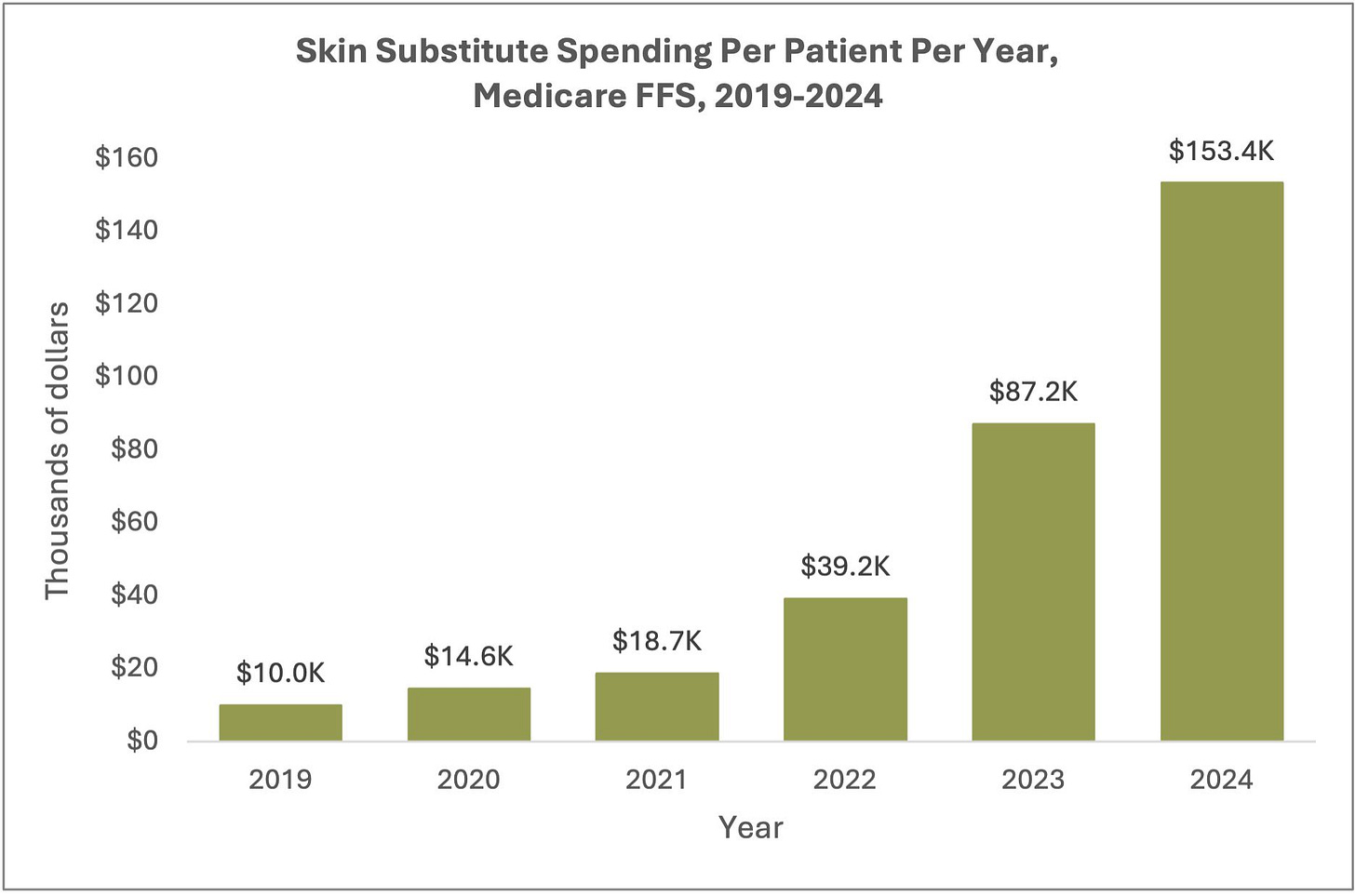
Medicare spending on skin substitutes surged from $200M to $10B in five years. An L&M Policy Research analysis of 2019–2024 Medicare FFS data found a 50-fold spending increase, driven by a 15-fold rise in per-patient costs, higher-priced products, and more claims per patient. The patient population more than tripled, with treatment increasingly shifting from offices to home and nursing home settings—prompting CMS to target skin substitutes in its new WISeR Model for reducing unsupported care (L&M Policy Research).
CLINICAL
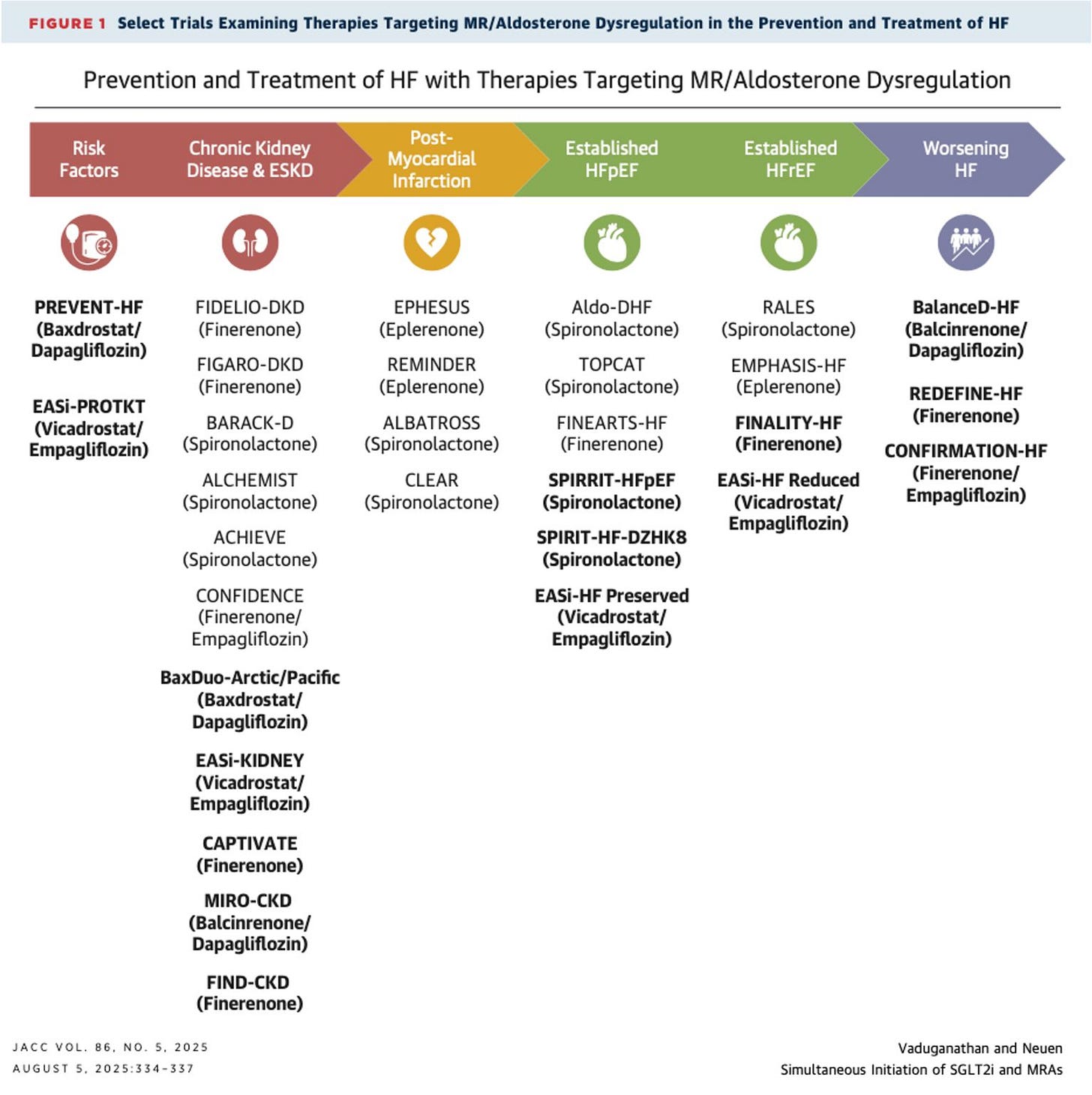
Optimizing SGLT2 inhibitor–MRA combination therapy in HFpEF and CKD. A JACC commentary reviews new trial data from SOGALDI-PEF and CONFIDENCE, highlighting strategies to safely implement dapagliflozin–spironolactone therapy in high-risk patients. The authors emphasize managing early eGFR declines and potassium changes, sequencing drugs to minimize risk, and addressing therapeutic inertia to ensure eligible patients benefit from treatment intensification across the cardio-kidney-metabolic spectrum (JACC, h/t Brendon Neuen).
Genomic biobanks drive new insights into kidney disease genetics. A Seminars in Nephrology review highlights how large-scale biobank data have identified key CKD risk genes, enabled polygenic risk scoring, and advanced understanding of monogenic disorders like ADPKD and Alport syndrome. Integrating multi-omics and expanding biobank diversity could improve early detection, risk stratification, and personalized treatment for CKD worldwide (SiN, h/t Alex Chang).
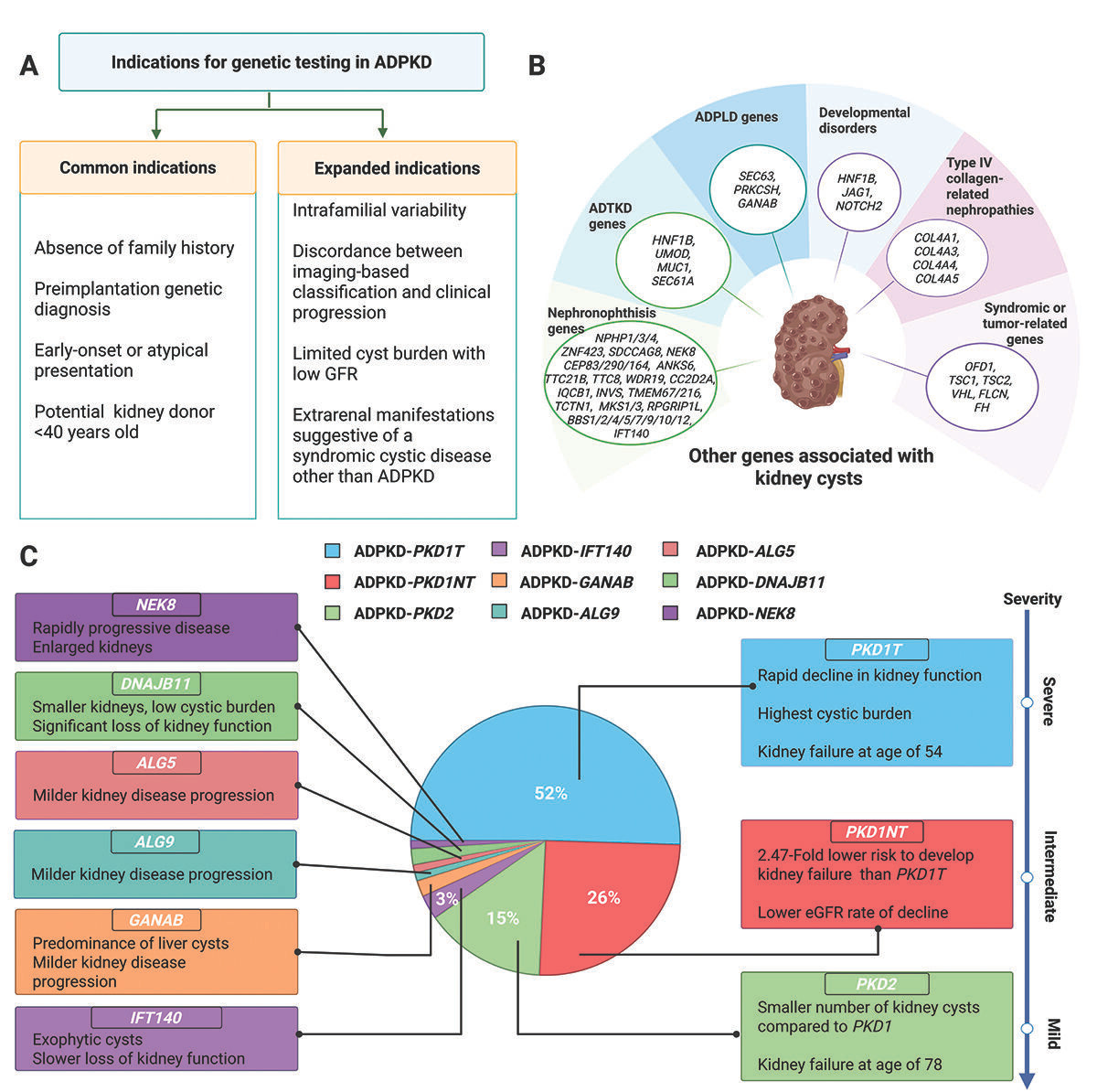
KDIGO issues first clinical guidelines for genetic testing in ADPKD. The 2025 recommendations expand genetic testing beyond atypical or early-onset cases to include situations like unexplained severity differences within families or imaging–progression mismatches. With PKD1 and PKD2 accounting for 93% of cases and six other genes linked to the phenotype, KDIGO now advises including the causal gene in disease nomenclature and using results for risk stratification (e.g., PROPKD score). Broader panels should also capture genetic mimics to improve diagnosis, prognosis, and readiness for emerging genotype-specific therapies (Kidney News, h/t Pranav Garimella).
Serum proteomics model improves ADPKD progression prediction. In a Nature study, researchers identified 29 proteins linked to annual kidney function decline, with functional pathways tied to immunity, lipoproteins, and metabolism. A final six-protein model—including SERPINF1, GPX3, and CFHR1—predicted outcomes independently of current clinical and imaging tools, with validation across cohorts supporting its potential for more accurate, individualized risk stratification. (Nature, h/t Roman-Ulrich Mülle).
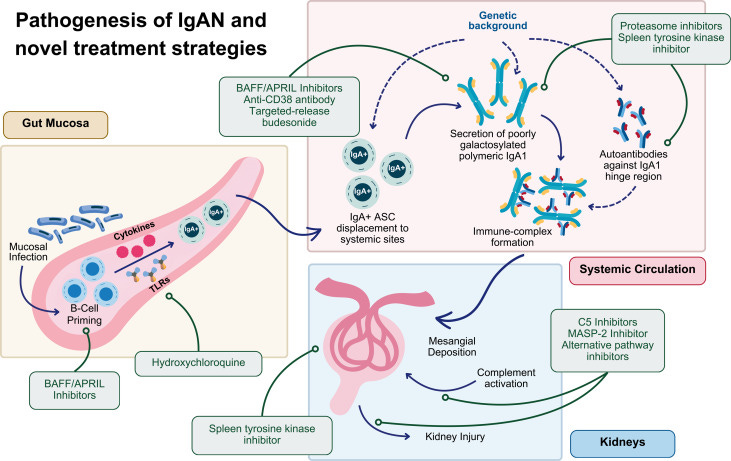
Clinical trials pipeline for IgA nephropathy continues to expand. A new review highlights how recent approvals—including delayed-release budesonide, sparsentan, atrasentan, and iptacopan—have transformed IgAN care, with ongoing trials targeting mucosal immunity, B-cell activation, and complement pathways. The authors note that proteinuria reduction is now widely accepted as a surrogate endpoint, opening the door for faster drug development and more tailored therapeutic strategies (Kidney Medicine, h/t Sayna Norouzi, MD, et al.).
eGFR combining creatinine and cystatin C improves accuracy across BMI extremes. In a study of 4,707 Swedish adults with measured GFR, indexed eGFRcr-cys outperformed creatinine- or cystatin C–only estimates for most BMI ranges, improving dosing and treatment eligibility decisions. Nonindexed eGFR further reduced error at BMI extremes, with the authors recommending nonindexed eGFRcys for very low BMI and nonindexed eGFRcr-cys for very high BMI to optimize accuracy in clinical care (JASN, h/t Edouard Fu).
TRANSPLANTATION
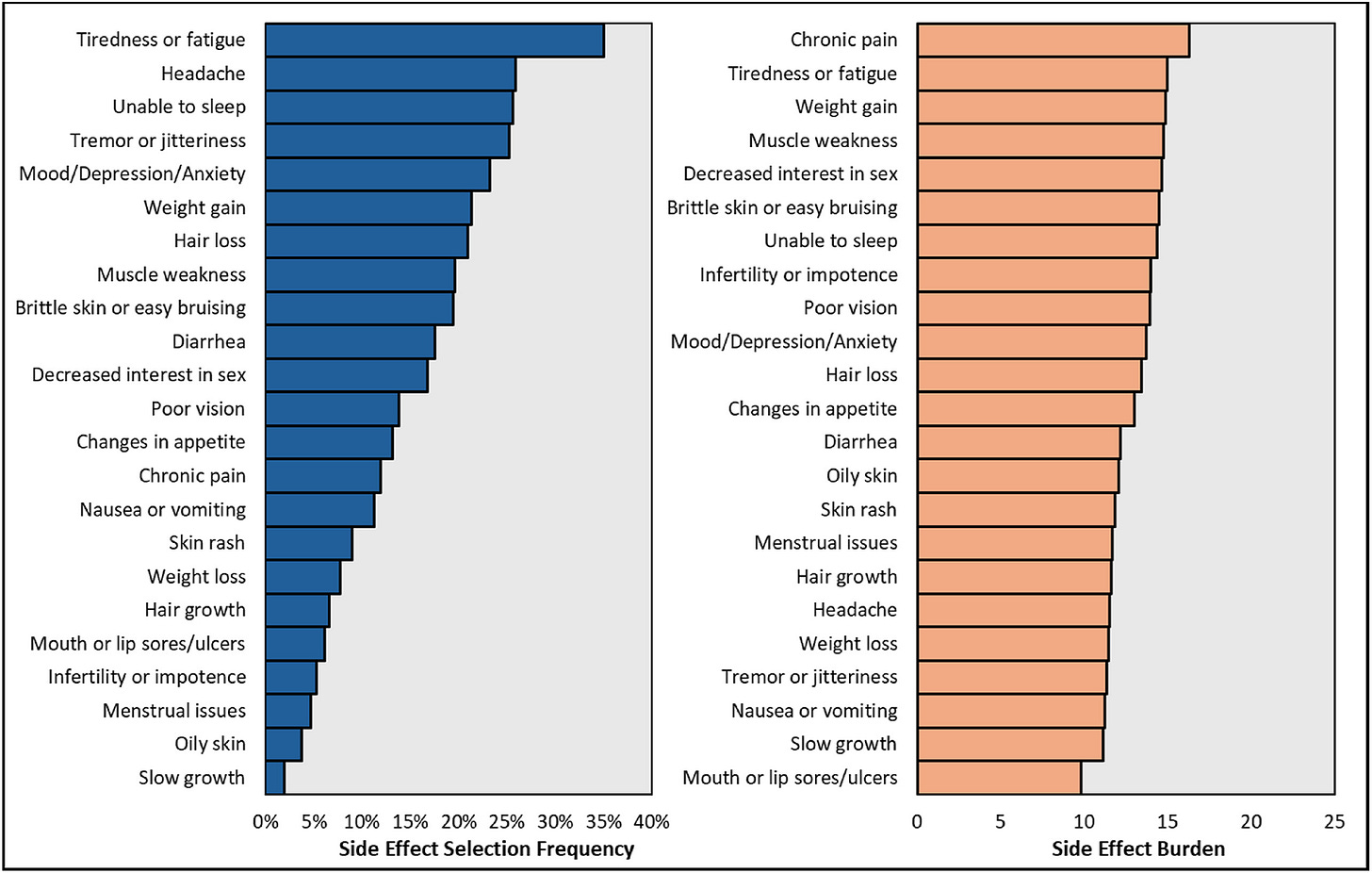
Survey finds heavy side-effect burden from immunosuppression in transplant recipients. In an AST survey of over 10,000 recipients across 232 US centers, 92% reported at least one side effect—most with moderate to high daily impact—including skin cancer, neuropathy, kidney disease, and cognitive issues. While trust and adherence were generally high, 25% skipped doses due to side effects and 40% due to costs, underscoring the urgent need for safer, more tolerable therapies (AJT).
Stem cell therapy enables kidney transplant patients to stop anti-rejection drugs. In a Phase 3 trial of MDR-101, donor-derived stem cells allowed 16 of 19 kidney transplant recipients with genetically matched sibling donors to discontinue immunosuppressants after two years. The approach, which reprograms the immune system through “mixed chimerism,” could extend kidney graft survival, improve quality of life, and reduce the need for repeat transplants, though broader applicability remains under study (NBC News, ClinicalTrials.gov).
DBD-eligible donors choosing the DCD pathway show reliable donation timelines and strong outcomes. A UK review (2012–2022) identified 123 cases where donors eligible for brain death donation instead proceeded via circulatory death, most often so families could be present at asystole. Time to cardiac arrest was predictably within acceptable limits, and organ utilization matched or exceeded standard DCD, with notably higher liver donation rates. Kidney graft outcomes were similar across groups, with signs of reduced delayed graft function compared to standard DCD donors (Transplantation).
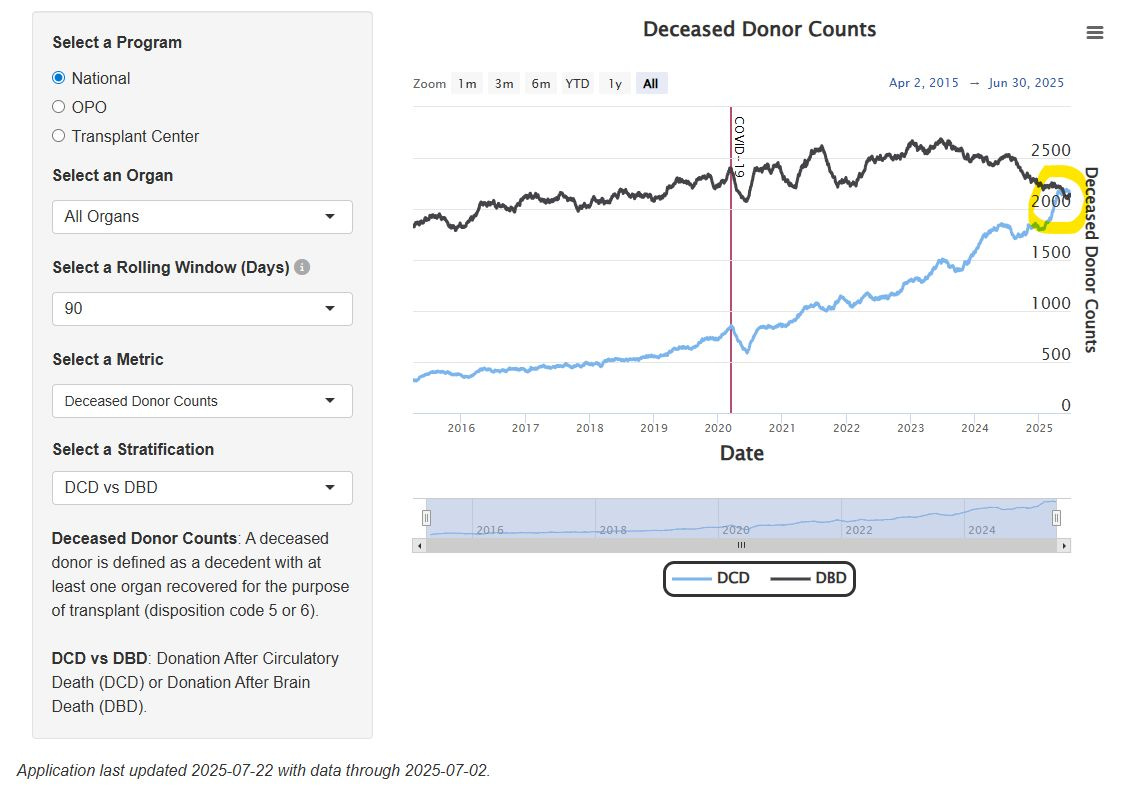
US sees first 90-day period with more DCD donors than DBD donors. SRTR data show a historic shift in organ donation patterns, with donation after circulatory death (DCD) outpacing donation after brain death (DBD) over a recent three-month span—a trend that could have implications for organ utilization strategies and transplant outcomes (SRTR, h/t Nicholas Wood).
Community
ADVOCACY
NCQA releases quality framework to close CKD care gaps. With CKD affecting 36M US adults—90% undiagnosed—NCQA convened a multidisciplinary panel to identify barriers and strategies for prevention, screening, and management. The resulting white paper outlines quality care elements, CKD’s role in the cardio-kidney-metabolic continuum, and actionable recommendations to inform future quality measures (NCQA, supported by Boehringer Ingelheim & Novo Nordisk).
Kidney patients see opportunity in RFK Jr.'s upheaval. The American Association of Kidney Patients is urging HHS Secretary Robert F. Kennedy Jr. to replace the U.S. Preventive Services Task Force, criticizing its resistance to expanding CKD screening despite new diagnostics and therapies. The group says screening should be standard for people with a family history, diabetes, or high blood pressure, while medical organizations warn that such a shake-up could politicize the panel and erode trust in its recommendations (Politico).
NKF supports new House strategy for Living Donor Protection Act. The National Kidney Foundation praised Representatives Bacon and Nadler for introducing two companion bills—H.R. 4582 and H.R. 4583—to advance insurance protections and FMLA coverage for living organ donors. Splitting the legislation aims to ease passage, building on NKF’s decade of advocacy and similar protections already enacted in over 30 states (NKF).
SEISMIC C3G & IC-MPGN Summit — NephCure’s recent two-day summit convened international experts, patients, and advocates to chart a path forward for rare kidney diseases C3G and IC-MPGN. Discussions focused on accelerating diagnosis, expanding use of biomarkers, building centers of excellence, and making disease-modifying therapy a first-line treatment. Workgroups will now begin implementing the actionable solutions identified during the meeting (NephCure).
Closing the distance: Fixing access to care in rural America. Writing in Forbes, Bill Frist outlines how nearly 60 million rural Americans face barriers beyond geography—including workforce shortages, facility closures, broadband gaps, and cost—that limit access to trusted, high-quality care. He calls for new rural-focused delivery and payment models, early pipeline programs like high school health career tracks, and expansion of non-physician provider roles. Frist highlights examples from East Tennessee State University, Meharry Medical College, and telehealth expansion as pathways to strengthen rural health ecosystems (Forbes, h/t Senator Frist).
Fresenius hosts Asia Pacific HOME nephrology meeting in Manila. Led by Dr. Harry Li, the event gathered over 50 participants from across Asia Pacific—including colleagues from Thailand, Hong Kong, Malaysia, Singapore, and the Philippines—for an intensive program on optimizing home dialysis therapies. Hosted by Country GM Mike Pangilinan, the meeting highlighted regional collaboration and shared commitment to improving patient outcomes at home (h/t Harry Li).
GOVERNMENT
ARPA-H backs autonomous robotic kidney surgery project at Johns Hopkins. The SARRTS initiative, led by Dr. Ileana Hancu, is developing supervised autonomous technology for renal tumor resection, aiming to enable general surgeons in rural hospitals to oversee complex procedures. In a recent demo, Dr. Axel Krieger’s team used a surgical robot to remove tumors from a “phantom kidney,” showcasing progress toward expanding access to high-quality oncology care. The team also published a Science Robotics review on the path from today’s teleoperated systems to fully autonomous surgical robots, highlighting AI-driven advances, levels of autonomy, and the potential to deliver safer, more consistent surgery anywhere—even in remote or space environments (ARPA-H, Science Robotics).
CMS Proposes 2026 ESRD PPS Updates. The 2026 proposed rule for the ESRD Prospective Payment System would raise the base rate to $281.06 (a 1.9% payment increase) for both ESRD and AKI patients. CMS also proposes shortening the ICH CAHPS survey from 62 to 39 questions—removing the nephrologist communication/caring measure and consolidating race/ethnicity questions—and eliminating three health equity reporting measures from the QIP beginning in PY 2027. Comments are due by September 12 (Federal Register, h/t Robert Blaser).
CMS launches initiative to expand patient-driven health data tools. Led by Amy Gleason, the program aims to build an ecosystem of apps—some AI-powered, others leveraging SMART Health Cards and QR codes—to give patients direct access to and control over their health data. Brian Anderson of CHAI called it a milestone toward making personal data use tangible and encouraged developers and CHAI members to join the effort (h/t Brian Anderson).
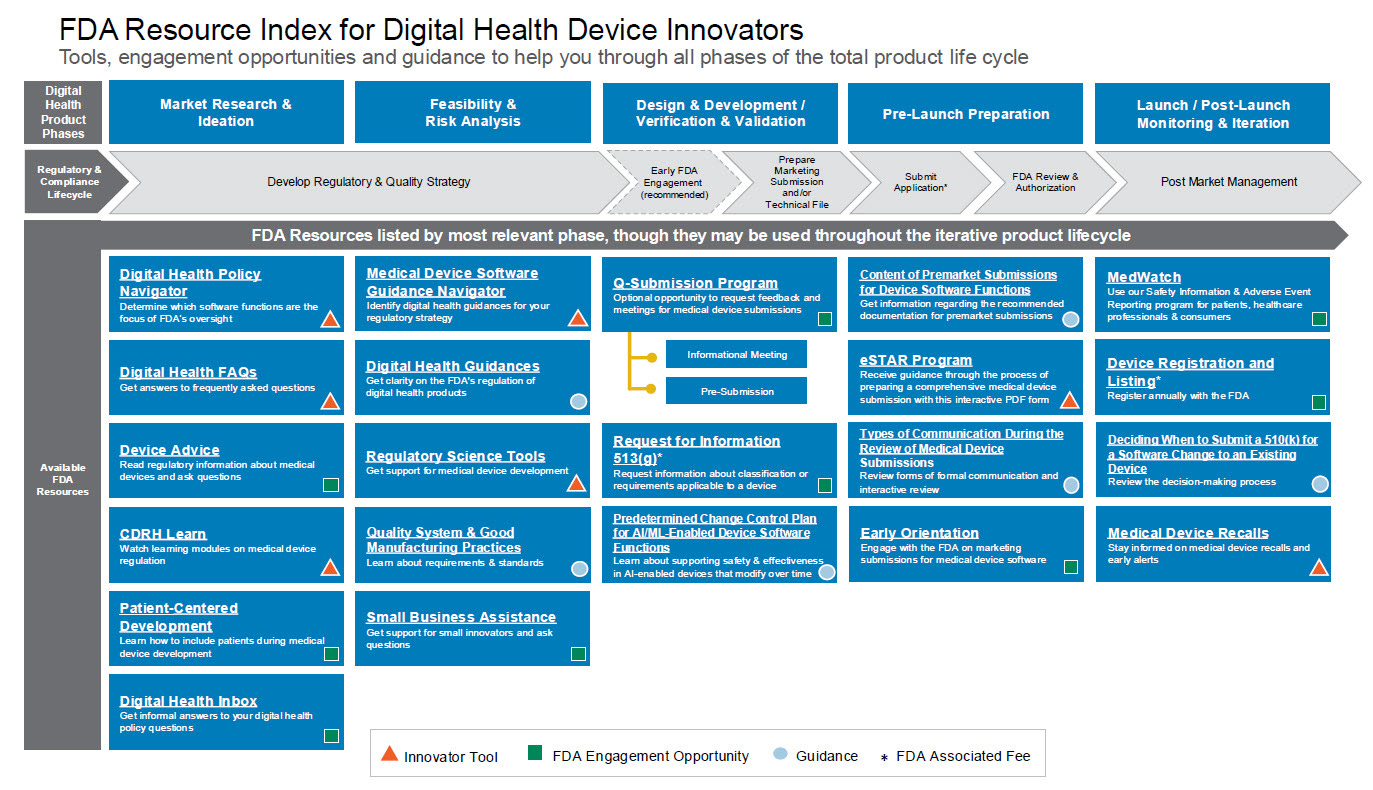
FDA launches Regulatory Accelerator for digital health innovators. The new program from the Digital Health Center of Excellence offers a curated “Resource Index for Innovators,” optional early-orientation meetings with FDA reviewers, and a Medical Device Software Guidance Navigator to help startups and developers map their regulatory path. Designed to simplify navigation, speed market entry, and improve submission quality, the Accelerator consolidates tools and guidance for medical device software across its full life cycle (FDA, h/t Yujan Shreshta).
WORLD TRANSPLANT CONGRESS
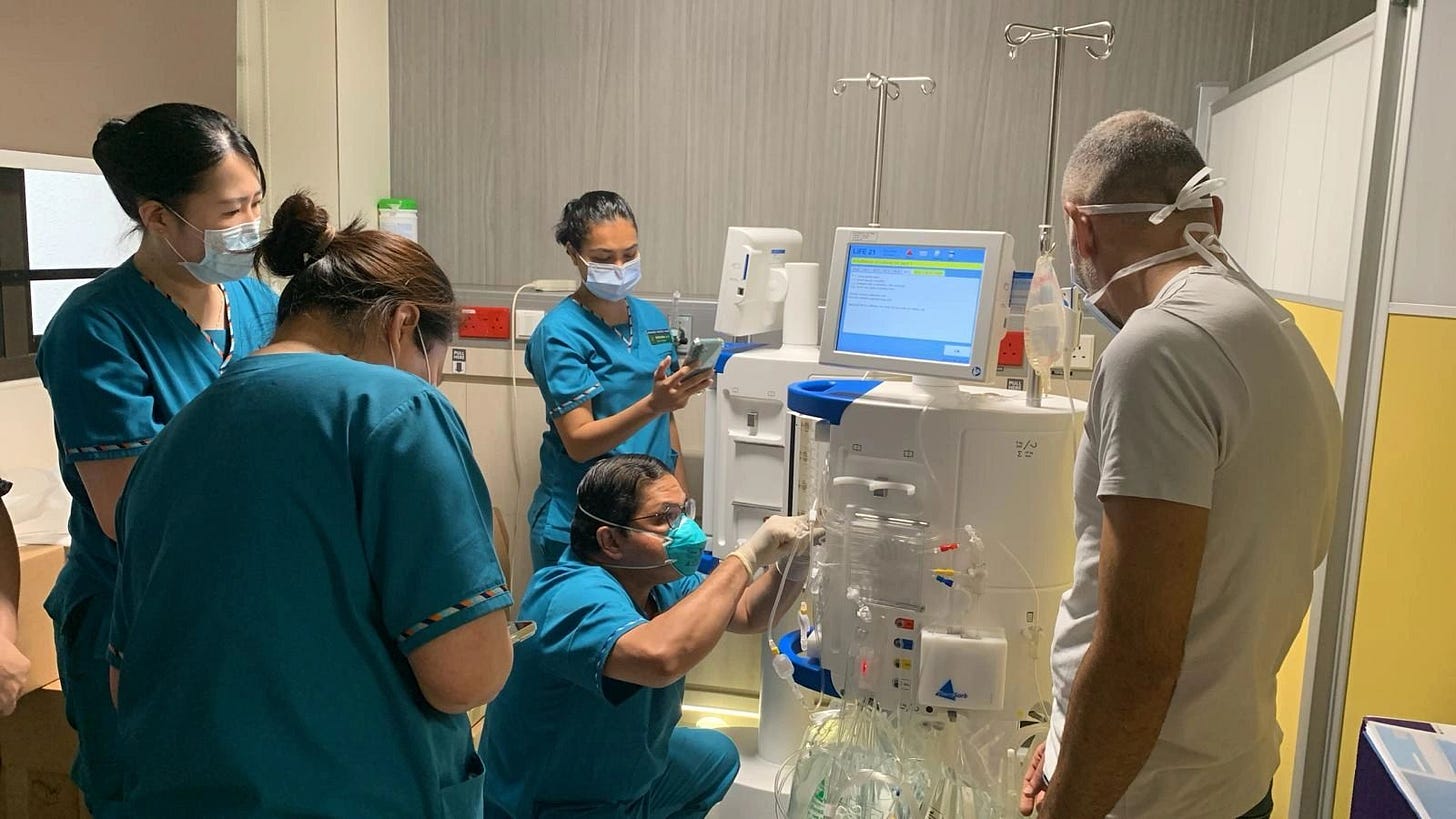
Singapore General Hospital advances kidney transplant care with regional firsts. At the World Transplant Congress, SGH’s transplant team—led by Director Terence Kee—presented research on cell-free DNA, pharmacogenomics, and gender disparities, reflecting their patient-centered focus and collaborative research in novel diagnostics, viral infections, and immunosuppression. The program also achieved the Asia-Pacific’s first use of the Therasorb immuno-adsorption system for desensitizing high-risk kidney transplant recipients, successfully reducing antibody levels to enable rejection-free transplantation (h/t Terence Kee).
Dr. Alexandre Loupy presented a new Virtual Biopsy System — a machine learning tool trained on data from 16,000 patients across 17 centers worldwide to predict histological graft lesions without an actual biopsy. Using only basic donor information, the ensemble ML model achieved high precision in predicting Banff scores, performed consistently across donor types and geographies, and could help standardize organ assessment and optimize allocation strategies (Nature, h/t Alex Loupy)
CareDx debuts AlloSure Plus and new transplant data at WTC 2025. In over 40 featured abstracts, studies underscored donor-derived cell-free DNA’s value in detecting rejection, predicting graft loss, and guiding surveillance. Validated in over 1,000 renal biopsies, the AI-powered AlloSure Plus integrates dd-cfDNA with clinical data for personalized rejection risk and will launch via EPIC Aura in Q3 2025. Highlights included an 8-fold higher graft loss risk with elevated dd-cfDNA, strong outcomes in high-risk kidney recipients, and enhanced prognostication using combined dd-cfDNA and gene expression profiling (CareDx).
BMI OrganBank secures FDA Breakthrough Device Designation for kidney preservation platform. The company’s room-temperature machine perfusion (RTMP) device enables real-time quality assessment and extended preservation times, aiming to increase usable kidneys and reduce waitlist times. Developed with transplant surgeons and academic centers, the portable system will enter clinical studies early next year (PR Newswire).
A wave of innovation is reshaping organ preservation and logistics. From Diatiro’s new KidneyPod (launched at WTC) and Northernmost’s NoMo system (unveiled at AOPO) to BMI OrganBank’s FDA Breakthrough-designated room temperature kidney preservation device, announced during WTC. Market leaders Paragonix (acquired by Getinge) and TransMedics (working on kidney transport), along with OrganOx and 34Lives (ARPA-H awardee), and Bridge to Life (whose new HOPE vs. NMP study at WTC showed improved graft and patient survival with fewer complications), are advancing technologies to improve transport, reduce discards, prevent delayed graft function, extend preservation times, and simplify surgery. All target a small but critical customer base of 56 OPOs and 250 U.S. transplant centers. We’ll be diving deeper into this market and problem space soon. are advancing technologies to improve transport, reduce discards, prevent delayed graft function, extend preservation times, and simplify surgery. All target a small but critical customer base of 56 OPOs and 250 U.S. transplant centers. We’ll be diving deeper into this market and problem space soon. Thanks to all of you who spent time with me during WTC, and congrats on your progress!
25 Questions for Kidney Transplant. As WTC kicked off in San Francisco last week, I shared 25 big questions shaping the future of kidney transplantation, from waitlist growth and living donation trends to governance reform, xenotransplantation, immunosuppression breakthroughs, and payment model innovation. It’s a personal mix of optimism and caution, aimed at sparking dialogue across science, policy, and patient care (Signals).
Events Calendar
Budapest Nephrology School (August 25-29)
SLANH 2025 (Guayaquil, August 27-30)
Russian Dialysis Society (Moscow, September 5)
OSNT International, Salalah 2025 (Oman, September 4-6)
LSI Emerging MedTech (London, September 7-10)
Women in Medicine Summit (Chicago, September 18-20)
ASN Kidney Week (Houston, November 3-6)
Jobs
Nephrologist (Miami, FL) — Kaüna (Say hello!)
AI Analyst (Paris, FR) — PITOR
Senior Engineer, Lean Manufacturing — Edwards
Lead Nephrology Specialist (West) — Monogram
Director, Clinical Operations — US Heart and Vascular
Senior Dir, Hema / Neph Patient Services — AstraZeneca
Senior Principal Engineer, Prod Quality — Vantive
Assoc. Director, Pharmacovigilance — Travere
Senior Director, Strategic Initiatives — Monogram
Transplant Account Manager — CareDx
Director, Health Plan Reporting & Analytics — Cityblock
Staff Systems Engineer — Mendaera
Nephrologist (Murray, UT) — Intermountain
Browse hundreds more at jobs.signalsfs.com
Work with us
Want to help shape what comes next in kidney health? Signals Group is expanding to support the growth of this community. Whether you’re preparing for a new milestone, growing awareness for your mission, or looking to enter a new market, we’d love to hear from you and support your journey.
Share — Tell us what unmet needs you’d like to see solved
Sponsor — Share your work with 15,000 kidney professionals
Subscribe — Support independent kidney news and research
Grow — Partner with us to reach your next company milestones
This audio summary may include variations in pronunciation and is intended for informational purposes only. For complete accuracy and source attribution, please refer directly to the original written materials and cited sources. Always consult trusted references when interpreting medical or scientific content.
![Signals From [Space]](https://substackcdn.com/image/fetch/$s_!IXc-!,w_40,h_40,c_fill,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F9f7142a0-6602-495d-ab65-0e4c98cc67d4_450x450.png)
![Signals From [Space]](https://substackcdn.com/image/fetch/$s_!lBsj!,e_trim:10:white/e_trim:10:transparent/h_48,c_limit,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F0e0f61bc-e3f5-4f03-9c6e-5ca5da1fa095_1848x352.png)
![Signals From [Space]](https://substackcdn.com/image/fetch/$s_!NnOt!,w_144,h_144,c_fill,f_auto,q_auto:good,fl_progressive:steep,g_auto/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F688fc47b-7202-4a2e-b4f4-fea2b047ab1b_1500x1500.png)