Most kidney doctors were never trained to use genetics in their everyday practice. But that’s changing fast. In this conversation, Dr. Andrew “Andy” Lazar of University Hospitals Cleveland shares how genetic testing has helped him uncover hidden causes of chronic kidney disease, connect patients to clinical trials, and better understand APOL1’s role in health disparities.
Dr. Lazar’s story builds on recent conversations with Bryce Powerman and Ronen Schneider, who highlighted how broad genetic testing and data-driven trial design are accelerating discoveries from bench to bedside. Together, they paint a picture of a field in transition, where the line between diagnostics and therapeutics is blurring, and where understanding a patient’s genes may be the key to preventing kidney failure altogether.
What’s Inside:
Dr. Lazar’s path from engineering to medicine
How genetics is reshaping clinical nephrology
Why identifying APOL1 variants matter for kidney care
What studies like RenaCARE and AASK show us
How data from Renasight IQ is driving new trials
Overcoming barriers to test adoption and patient trust
Q&A
Dr. Lazar, tell us a bit about your background.
AL: I actually began my career as a biomedical engineer, working on fluid-dynamics problems for the automotive industry. Torque converters, cylinder-head designs, that sort of thing. I always knew I wanted to go to medical school but wasn’t quite ready at 21. After several years with General Motors, I went to medical school in Michigan. I later trained at the University of Virginia and Case Western Reserve, focusing on renal disease.
Today I’m a practicing nephrologist at University Hospitals Cleveland. I still see patients weekly, but over time I’ve become deeply involved in clinical trials, especially around lupus and other inflammatory kidney diseases. My engineering roots came full circle: I now also work on an implantable dialysis device, an early form of the “artificial kidney.” But at the center of everything is still patient care, so that’s what I get excited about and why I’m here.
When did genetics become part of that journey?
AL: About five or six years ago. During training, genetics wasn’t a major focus, and I realized there was a gap in my own understanding. Around 2020, I began seeing more and more landmark papers in The New England Journal of Medicine and JASN connecting genetics to kidney disease.
I decided not to shy away from it. I started ordering genetic tests, initially through Natera’s Renasight panel, which covers nearly 400 genes linked to kidney disorders. What I found was eye-opening.
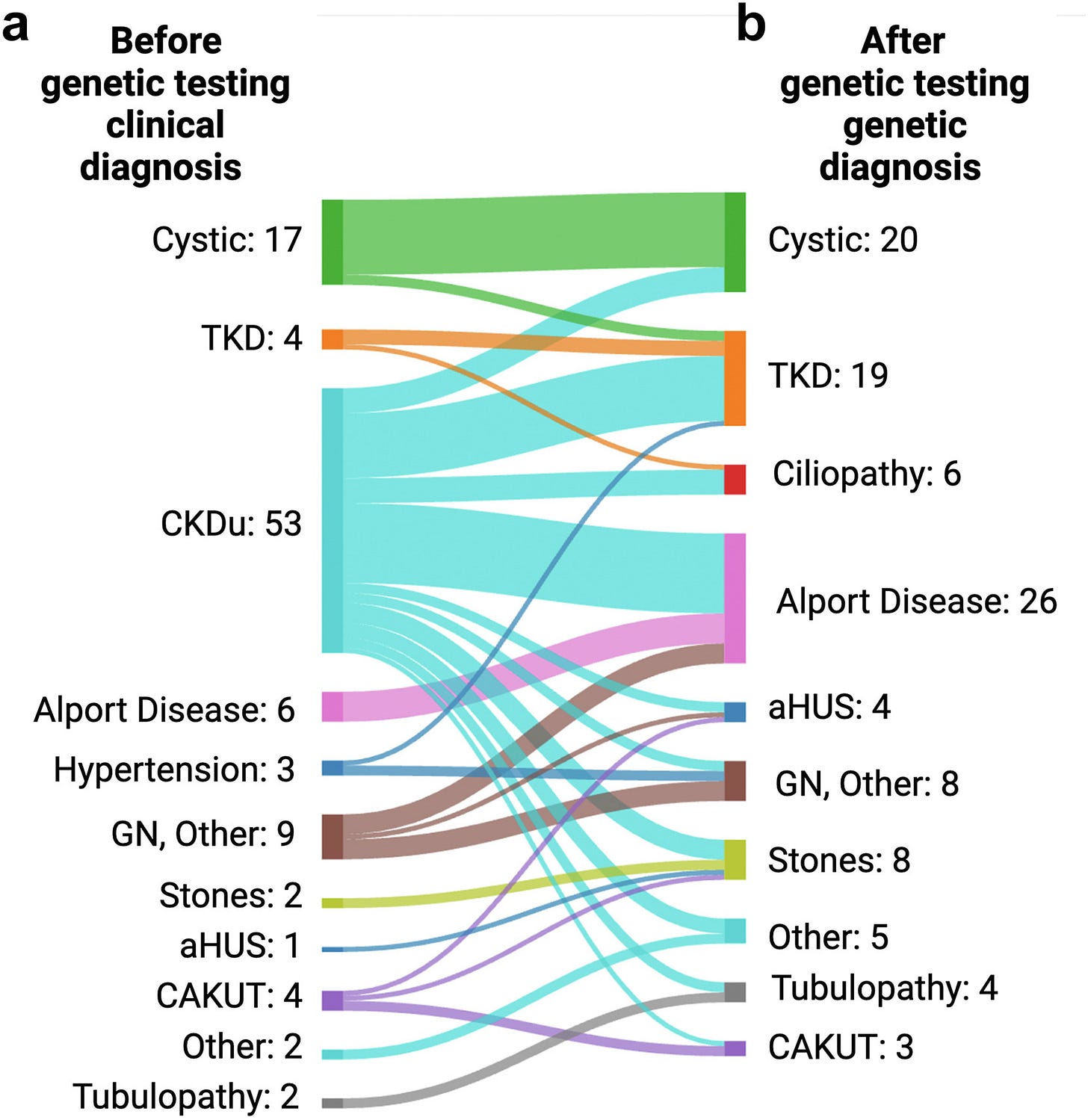
Roughly one in five adults with chronic kidney disease likely has a genetic cause. Sometimes it’s a single-gene (“monogenic”) change; other times multiple variants interact. Many of these patients had been labeled “hypertensive nephrosclerosis” or “CKD of unknown etiology (CKDu).” Once I began testing, I started getting real answers for people I’d followed for years.
And that changed your clinical practice?
AL: Completely. Some of those findings pointed to diseases we can now treat or enroll in trials. Conditions like Alport syndrome or APOL1-mediated kidney disease.
At first I was just “dipping my toe in the water,” but genetics quickly became part of my everyday workflow. Tests that used to feel experimental are now routine and surprisingly affordable—often zero out-of-pocket for patients.
I began to see this pattern show up: about 1 in 5 patients tested had a single-gene explanation for their kidney disease. For most kinds of testing that’s an enormous yield. For context, I order many other expensive tests (e.g. autoimmune panels, myeloma work-ups) that are almost always negative. So a 20 percent hit rate is extraordinary.1
It sounds like several studies have helped quantify that. Could you walk us through what the data show?
AL: A 2019 New England Journal of Medicine paper looked at more than 3,300 CKD patients (about two-thirds were already on dialysis) and found 9.3 percent had a single-gene mutation explaining their disease, even without any clinical clues.2
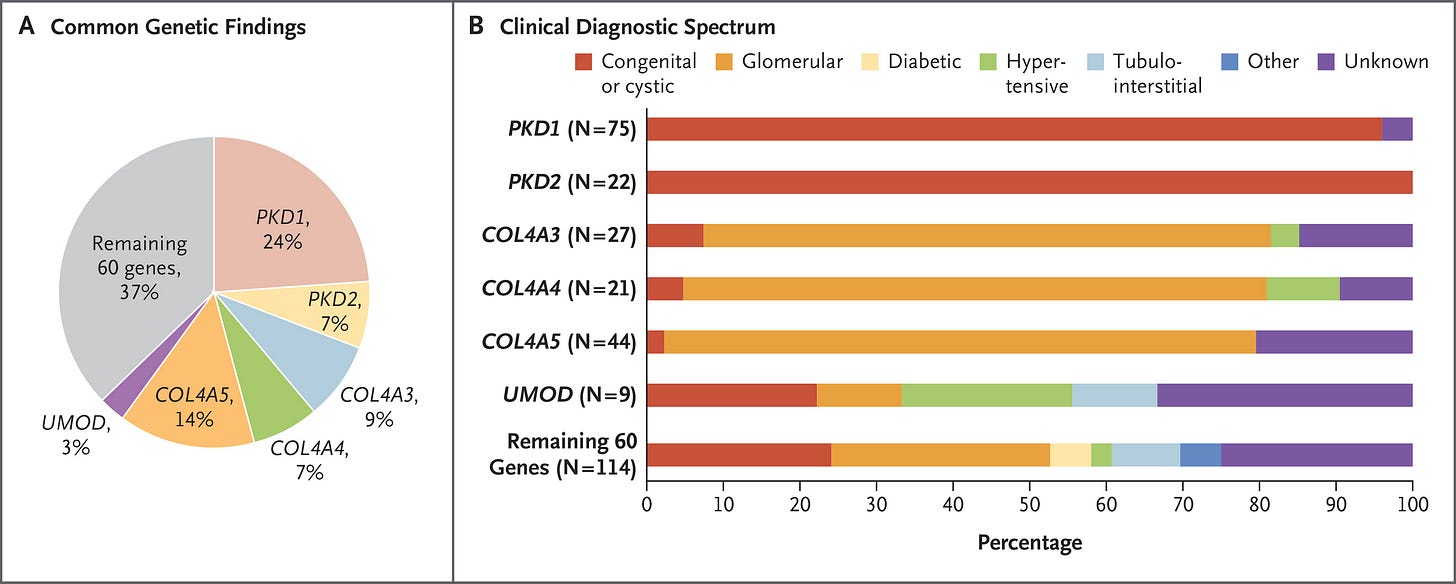
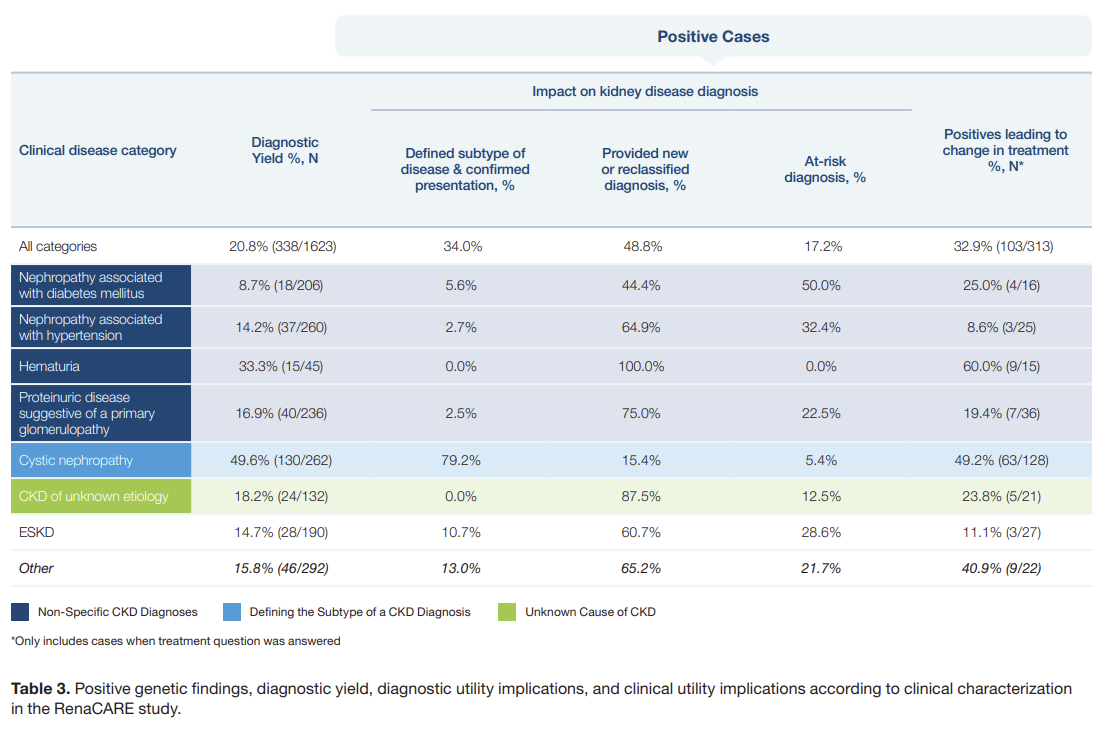
Subsequent studies like the RenaCARE trial and others in general nephrology clinics found yields closer to 20 percent, and in genetics-focused centers it can reach 40 percent when broader sequencing is used.3
Think about that: up to two in five patients walking into a CKD clinic may have an identifiable genetic driver. Yet most never get tested. But perhaps most importantly, around half of patients who received a positive genetic test result ended up having a change in their treatment plan. That simply does not happen without a test.
Figure: Disease Reclassification Before & After Testing
Let’s talk about APOL1. Why is this one so important?
AL: APOL1 stands for Apolipoprotein L1. It’s a gene that produces a protein originally meant to protect us. During human evolution in West Africa, certain versions of APOL1 (called the G1 and G2 variants) provided a survival advantage by destroying the parasite that causes African sleeping sickness (trypanosomiasis).
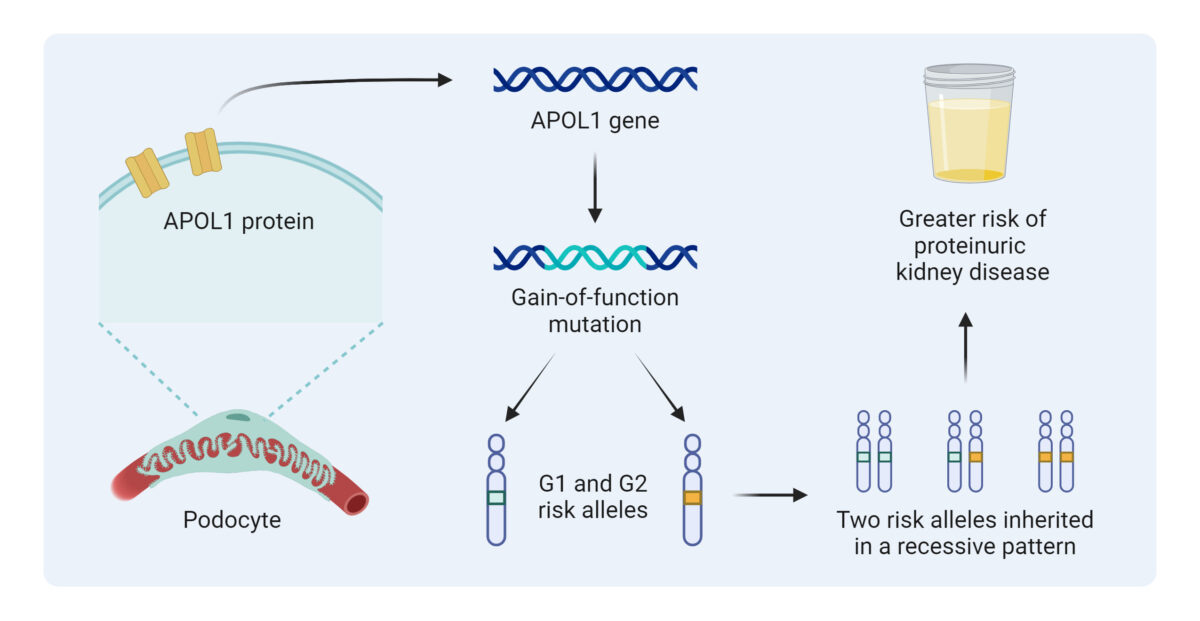
That’s what we call a gain-of-function mutation: the protein gained the ability to puncture holes in the parasite’s membrane (acting like a perforin channel). Unfortunately, that same property can also damage the kidney’s filtering cells, podocytes, leading to scarring known as FSGS (focal segmental glomerulosclerosis).
It’s a double-edged sword: protection from infection, but a higher lifetime risk of kidney failure. Today, about 13 percent of African Americans carry two risk variants of APOL1. Most have never been tested, yet around 20% of them will develop kidney disease in their lifetime.4
That’s staggering. Does this impact the way you think about treatment and trial design?
AL: Yes, it absolutely does. It means we can finally target the root cause. Multiple companies are developing therapies aimed at neutralizing APOL1’s harmful effects. Vertex Pharmaceuticals has a small-molecule inhibitor designed to block that perforin channel, and Maze Therapeutics is exploring RNA-based approaches to silence the gene’s expression.
The science is there, but trial enrollment is lagging. We have the potential to keep people off dialysis, yet if patients aren’t tested, they never know they’re eligible. Building trust in affected communities is essential. We cannot move the field forward without participation.
When you order these tests, how broad do you go?
AL: Very broad. That’s the idea. I typically use the 397-gene Renasight panel. Narrow panels can miss unexpected findings due to what’s called phenocopy, when one gene mimics the effects of another.
For example, if you test only 13 genes for a suspected disorder, you might detect 12 percent of cases. Expand to 55 genes and that yield jumps to 30 percent; go to ~400 and it can exceed 60 percent. So humility is key: order broadly, because biology rarely fits neat categories.
So then how does this influence the way you think about “common” causes of kidney disease, like hypertension or diabetes?
AL: It’s been humbling. For decades, we’ve labeled many patients as having hypertensive or diabetic kidney disease when genetics tell a different story.
In the historic AASK trial (African American Study of Kidney Disease and Hypertension), researchers found that standard therapies like ACE inhibitors didn’t slow kidney decline in some patients. We now know many of those individuals had APOL1-associated disease, not classic hypertension-related damage.5
Similarly, studies of diabetics with kidney disease show that 30 to 60 percent may have non-diabetic causes when biopsied. So when things don’t add up (e.g. normal eye exams, short diabetes duration, preserved A1C) it’s time to think genetics.
Figure: Positive Findings, Diagnostic Yield, & Utility
Where does real-world data come into play?
AL: Renasight IQ combines genetic and clinical data from more than 170,000 patients. It lets us see how genotype and therapy intersect.
For example, when we looked at 93,000 people with hypertension, those carrying two APOL1 risk variants lost kidney function fastest. But if they were on an SGLT2 inhibitor, their decline flattened dramatically, matching the “low-risk” group.
That’s observational, not causal, but it mirrors what we saw in randomized trials. Interestingly, ACE/ARB therapy didn’t help in high-risk APOL1 patients, consistent with AASK. For diabetics, GLP-1 agonists seemed protective. These kinds of datasets help us refine hypotheses and design smarter studies.
That’s a great segue to clinical-trial strategy. Will genetics change the way we design studies?
AL: It’s transformative. If you and I were developing a therapy for APOL1 disease, we’d want to enrich the trial population with patients who have those variants. That increases the likelihood of demonstrating benefit and reduces noise.
It’s exactly what oncology did two decades ago: selecting patients based on their tumor’s genetic profile. Nephrology is finally catching up, using genotype to match the right patient to the right intervention.
What other examples should we be following?
AL: You can’t walk into an oncologist’s office today without having your tumor sequenced. Treatment decisions hinge on it.
In kidney care, we’re still behind, but we can get there. The roadmap is clear: widespread testing, integration into electronic health records, and linking genetic findings to available trials. If we do that, we’ll move from reactive to precision medicine, just as oncology did.
Figure: Genetic test ordering frequency and its relationship with genetics education among US nephrologists
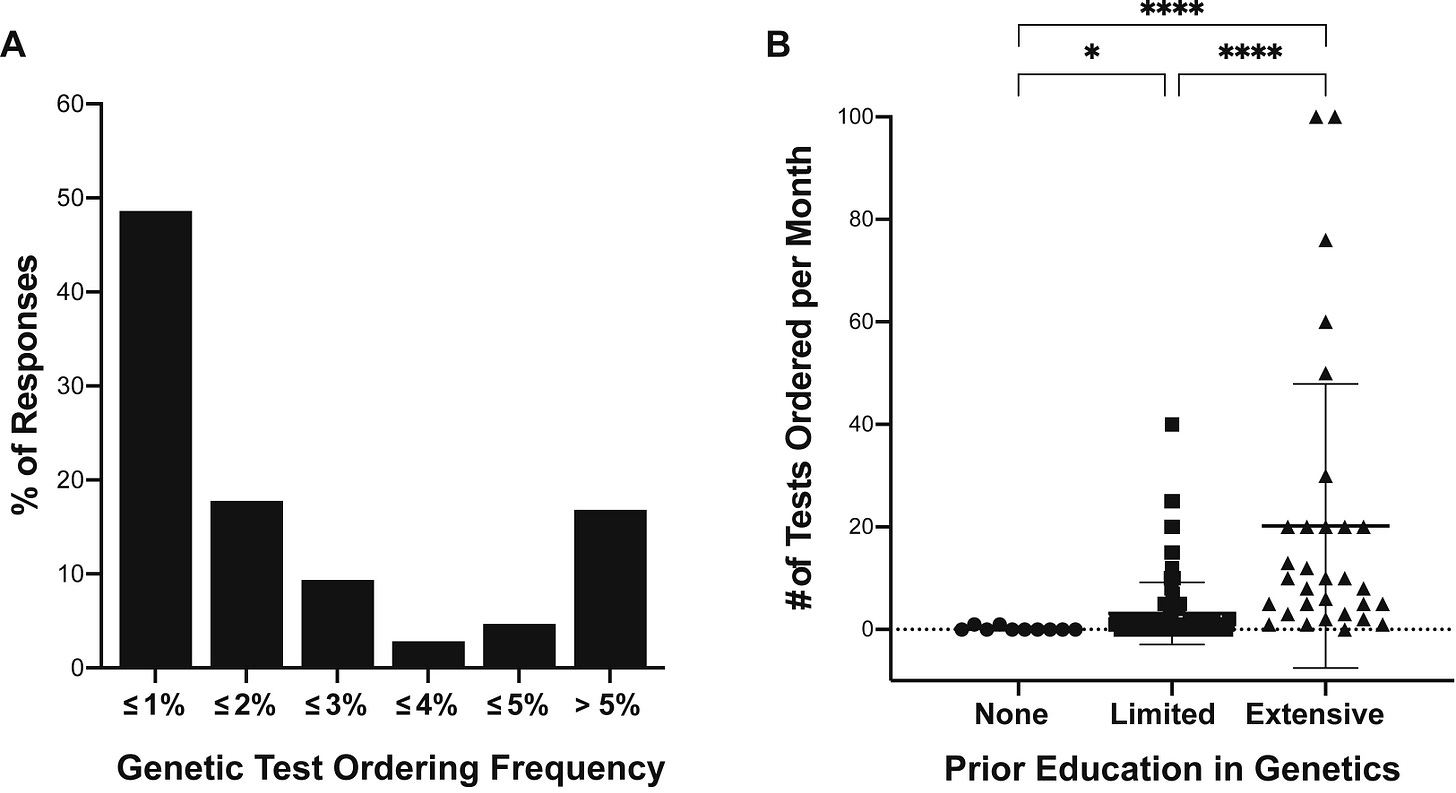
What’s standing in the way right now?
AL: Honestly, it’s us—health-care providers. Patients rarely refuse testing once it’s explained. The hesitation comes from clinicians who feel unprepared to interpret results or worry about cost.
But cost is no longer a real barrier. In my own practice, out of hundreds of tests, only a handful of patients have paid more than $99. Companies like Natera provide genetic-counseling support for both clinicians and patients. The resources are there; we just need to use them.
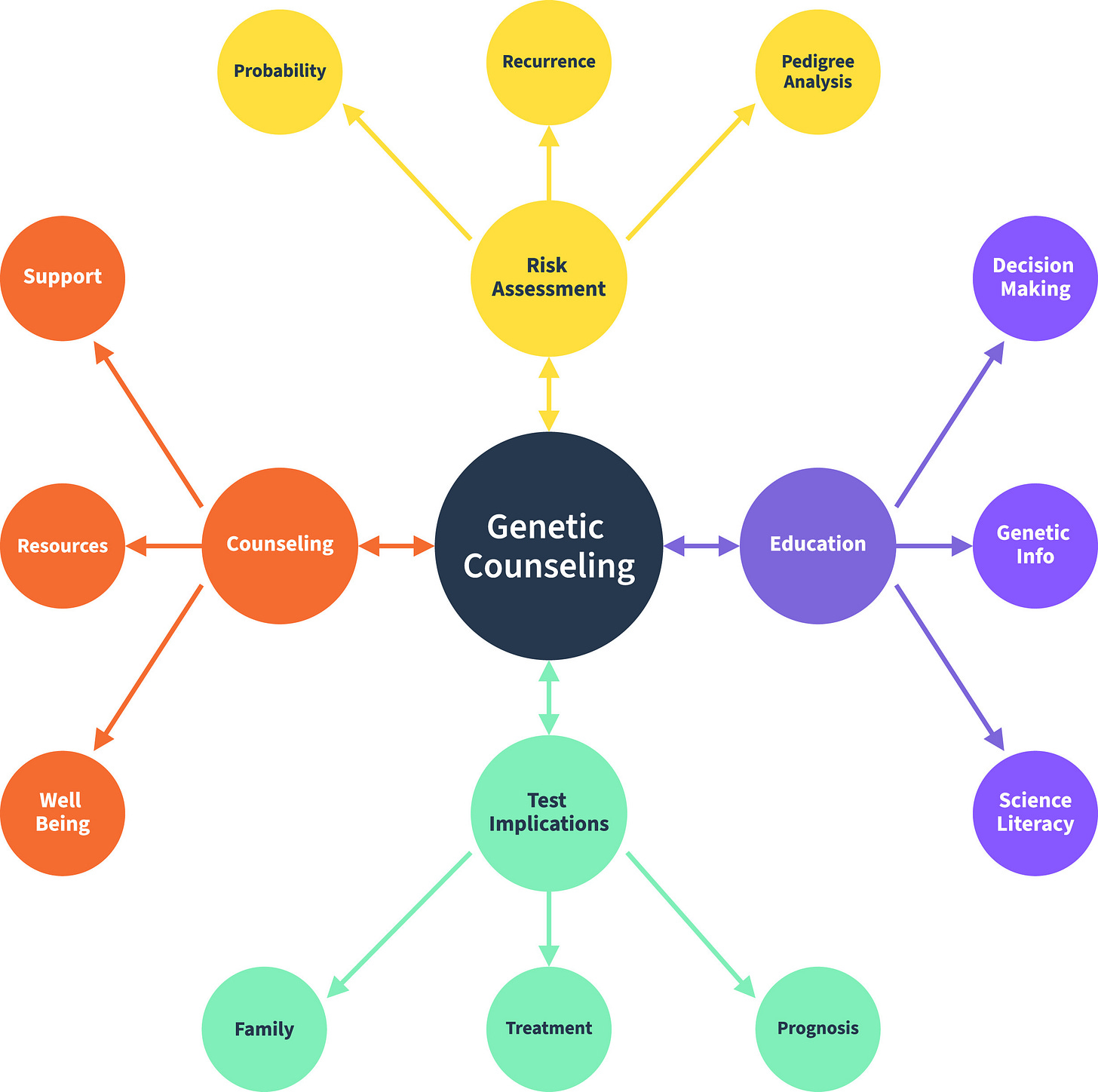
Figure: Components of genetic counseling6
Let’s talk about the human side. Any stories you can share?
AL: One that stays with me is a young man from Scotland. He’d been told for decades he had IgA nephropathy based on an old biopsy. His disease kept progressing and the details never quite fit. When Renasight became available, I ordered it. The result showed Dent’s disease, a rare X-linked disorder of proximal renal tubular dysfunction.
That discovery didn’t just change his management; it gave his family peace of mind. He and his wife had two sons and were terrified they might inherit it. Because the mutation is X-linked, it passes through the mother, so his boys were safe. There were tears of relief all around, myself included. Moments like that remind me why this matters and just what we can do.
Looking ahead, what’s your call to action for fellow clinicians and patients?
AL: For clinicians: I’d say fight to make a diagnosis. If a patient has unexplained protein or blood in the urine, a family history of kidney problems, or is of African descent with CKD, order a genetic test. The information can change lives and open trial opportunities.
For patients: we need to earn back your trust, but don’t be afraid to ask questions. Genetic testing isn’t about labels: it’s about clarity, empowerment, and options for your future.
We’re on the verge of breakthroughs for APOL1 disease and others. But to cross that finish line, we need participation: clinicians who test and patients who enroll. That’s how we’ll finally move kidney genetics from promise to proof.
My thanks to Dr. Lazar for joining us to share his story. For more on the power of genetic testing in kidney disease, see the 2023 JASN RenaCARE study, where 1 in 5 patients tested positive for a genetic cause of CKD and 1 in 3 had a change in treatment. The ENYO case study shows how Natera’s patient-matching platform accelerated trial enrollment for Alport syndrome by 3x: completing in 5 months versus the 16-month industry average. A 2024 collaboration with the Alport Syndrome Foundation highlighted common COL4 gene variants and the evolving role of precision diagnostics in rare kidney disease. In 2022, the Renasight 1000 study revealed that 25% of early patients tested had a genetic finding, with nearly half carrying rare variants not detectable with limited panels.
Schott C, Arnaldi M, Baker C, et al. Implementation of a kidney genetic service into the diagnostic pathway for patients with chronic kidney disease in Canada. Kidney Int Rep. 2025;10(2):574-590. doi:10.1016/j.ekir.2024.12.019
Westemeyer, Margaret; Bhorade, Sangeeta; Tabriziani, Hossein; Clark, Dinah. The RenaCARE Study: Updating Genetic Testing Results in Response to New Gene-Disease Association and Variant Upgrade: FR-PO658. Journal of the American Society of Nephrology 35(10S):10.1681/ASN.2024q0fwpe0h, October 2024. | DOI: 10.1681/ASN.2024q0fwpe0h
Datta S, Antonio BM, Zahler NH, et al. APOL1-mediated monovalent cation transport contributes to APOL1-mediated podocytopathy in kidney disease. J Clin Invest. 2024;134(2):e172262. doi:10.1172/JCI172262
Wright, Jr JT, Bakris G, Greene T, et al. Effect of Blood Pressure Lowering and Antihypertensive Drug Class on Progression of Hypertensive Kidney Disease: Results From the AASK Trial. JAMA. 2002;288(19):2421–2431. doi:10.1001/jama.288.19.2421
Stein Q, Westemeyer M, Darwish T, Collett K, Raible D, Hendricks E. Genetic counseling in kidney disease: A perspective. Kidney Med. 2023;5(7):100668. doi:10.1016/j.xkme.2023.100668
![Signals From [Space]](https://substackcdn.com/image/fetch/$s_!IXc-!,w_40,h_40,c_fill,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F9f7142a0-6602-495d-ab65-0e4c98cc67d4_450x450.png)
![Signals From [Space]](https://substackcdn.com/image/fetch/$s_!lBsj!,e_trim:10:white/e_trim:10:transparent/h_48,c_limit,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F0e0f61bc-e3f5-4f03-9c6e-5ca5da1fa095_1848x352.png)
![Signals From [Space]](https://substackcdn.com/image/fetch/$s_!NnOt!,w_144,h_144,c_fill,f_auto,q_auto:good,fl_progressive:steep,g_auto/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F688fc47b-7202-4a2e-b4f4-fea2b047ab1b_1500x1500.png)