Welcome to our first Signals roundup of the new year. If you recall, our last update spoke of potential energy building in kidney disease in the second half of 2025. Regulatory wins. New approvals. Promising data. Major media attention. For the first time in a long while, it felt like kidneys were finally being treated as a major organ system among their proximal peers. Our focus now is turning that potential into something real and lasting. I still hold two truths at once: kidney remain overlooked, and their moment has arrived. That tension excites me to no end, because that new narrative will change the world we know it.
You can see it in plain sight. Kidneys are showing up in places they rarely did before. A New York Times deep dive on metabolic health. Cardio–kidney–metabolic disease becoming a core pillar of CMS’ actions and innovation models. Other specialties increasingly looking to the kidney for answers to their own hardest problems.
The era of overlooking kidneys is ending. Now comes the work of showing why this elegant, underestimated organ is worth understanding, investing in, and fighting for.
That work won’t resolve overnight. It will take years. But our role is simple: make sure the right people are in the room when the future of kidney health is being shaped. If we do that well, the downstream impact will touch hundreds of millions of lives.
Days like this remind me why I started writing Signals in the first place. To cut through the noise. To connect people and ideas that actually move the field forward. And to help make progress in this “space” feel a little more concrete.
You’re a bigger part of that future than you might think. Thanks for being here.
We also released three new pieces this month, including Part 3 of our kidney value-based care landscape series, our list of 26 signals shaping the space this year, and a powerful guest essay by Jeff Parke on the hidden costs of care burden.
Have tips or feedback? Hit reply or drop us a note here.1
Reading time: 35 minutes
What’s Inside
News: CKM screening goes mainstream, AI platforms move into healthcare infrastructure, gene therapy momentum in rare kidney disease, transplant system scrutiny intensifies, CMS and FDA signal faster paths for health tech
Research: Kidney–heart cross-talk via extracellular vesicles, social determinants reshaping global kidney disease, disease-modifying signals in Alport syndrome, metabolic dysfunction as a root driver of CKD and CVD
Community: ACCESS model readiness and next steps, record-setting transplant volumes at Emory, in-home value-based care outcomes, new playbooks for tech-enabled kidney care, resources for builders and operators
Events: ASTS Winter Symposium, Mayo Clinic Clinician Update, AHIP: AI In Kidney Care, World Congress of Nephrology, RPA, and more
Jobs: State of Maine, Harvard, Vertex, Ardelyx, Travere, MediBeacon, Vantive, Primary Venture Partners, GEHA Health + dozens more on jobs.signalsfs.com…
This issue is made possible by Renalytix. Renalytix is an artificial intelligence-enabled in vitro diagnostics company, focused on optimizing clinical management of kidney disease to drive improved patient outcomes. Renalytix has received FDA approval and Medicare reimbursement for kidneyintelX.dkd which is offered commercially in the United States. Learn more about the test below, and watch my recent interview with Chief Medical Officer and Lab Director, Michael Donovan.
News
EARLY SCREENING & TX
Nina Agrawal’s recent piece in the New York Times unpacks how poor metabolic health, driven largely by excess visceral fat and insulin resistance, fuels cardiovascular disease, chronic kidney disease, diabetes, and even cancer. With the American Heart Association estimating that ~90% of U.S. adults have some degree of cardio-kidney-metabolic syndrome, the article underscores why early screening, prevention, and sustained lifestyle and medical interventions matter long before organ damage becomes irreversible.
Boehringer Ingelheim and CommonSpirit Health launched the CARES program, a community-led initiative to improve cardio-kidney-metabolic (CKM) screening and diagnosis in vulnerable populations, beginning in Chattanooga, TN and Omaha, NE. The collaboration focuses on increasing adherence to evidence-based guidelines through clinician training and locally co-created solutions that reflect community needs. CARES targets early identification of CKM conditions such as chronic kidney disease, diabetes, and cardiovascular disease, where under-screening and delayed diagnosis remain persistent challenges, particularly for Black and Hispanic/Latino communities. The partners describe the program as a scalable blueprint for health systems seeking sustainable, equity-driven approaches to CKM care. (Press release)
Carna Health raised $8M to scale global CKD screening following a strategic partnership with Siemens Healthineers. Together with NeoDocs, the group screened 35,000 people in Cameroon, showing how large-scale, low-cost detection can surface silent disease. Early CKD identification remains a top priority globally, and scalable screening models are finally moving from pilots to population impact. (H/t Salvatore Viscomi)
NEW THERAPIES / R&D
ENYO Pharma reported encouraging Phase 2 results for vonafexor in people with Alport syndrome, a rare genetic kidney disease with few treatment options. In their Alpestria-1 study, the drug reversed the typical decline in kidney function, shifting patients from a historical loss of kidney function to an average improvement while on treatment. Notably, most patients maintained lower protein levels in their urine even 3 months after stopping the drug, suggesting a potential disease-modifying effect rather than short-term symptom control. Based on these results, ENYO plans to move vonafexor into Phase 3 trials.
SonoThera launched a $125M Series B to move multiple genetic medicine programs toward first-in-human trials, including kidney diseases like ADPKD and X-linked Alport syndrome. The company’s nonviral RIPPLE™ ultrasound-based delivery platform has shown efficient delivery of DNA payloads to key kidney cell types, including tubular epithelial cells and podocytes, in non-human primate studies. The technology is designed to deliver large genetic payloads safely, repeatedly, and with targeted distribution across organs. SonoThera plans to begin Phase 1 human trials as early as 2027, marking a transition from preclinical development to clinical execution.
AI IN HEALTHCARE
Anthropic launched Claude for Healthcare, a HIPAA-ready suite of AI tools for providers, payers, life sciences teams, and patients, building on its latest Opus 4.5 model with stronger medical and scientific performance. The offering supports use cases like prior authorization, care coordination, regulatory submissions, and clinical trial operations, with connectors to CMS coverage databases, ICD-10, NPI registries, and major research platforms. U.S. Claude Pro and Max users can also opt in to connect personal health records to summarize data and explain results, with explicit privacy controls and no training use. The launch comes days after OpenAI announced ChatGPT Health. The race to define AI-native healthcare infrastructure is well underway.
OpenAI launched ChatGPT Health in the U.S., allowing users to upload medical records and health app data to receive more personalized health-related explanations and insights. OpenAI says health conversations are stored separately, won’t be used to train models, and are intended to support (not replace) medical care. Privacy advocates welcomed the potential benefits but warned that health data is highly sensitive and requires airtight safeguards, especially as AI companies push toward deeper personalization and new business models like advertising. The feature is rolling out to a small group of U.S. users and is not yet available in the UK or EU. (Press release, BBC News)
OpenAI acquired Torch, a four-person health records startup, in an acqui-hire reportedly valued at around $100 million in equity. Torch was building a way to unify medical records, labs, wearables, and consumer health data into a “medical memory for AI”. The team formerly worked together at Forward Health (former member here, I do miss it), and will now join OpenAI’s newly announced ChatGPT Health initiative. (TechCrunch, Axios)
Author’s note: if you are interested in the topic of care navigation, you might enjoy my recent deep dive on what kidney can learn from cancer care here, or my recent conversations with Intermountain and ChenMed on their kidney navigation service models.
POLICY / REGULATORY
ARPA-H launched ADVOCATE, a new program to develop FDA-authorized agentic AI systems capable of delivering specialty cardiovascular care in regions with severe clinician shortages, where 46% of U.S. counties lack a cardiologist. The initiative aims to fund full-stack AI solutions that can autonomously manage advanced heart disease care, alongside supervisory AI systems to monitor safety and performance after deployment, and partnerships with health systems to ensure real-world adoption. ARPA-H positions ADVOCATE as a potential blueprint for addressing clinician shortages across chronic diseases, with an independent analysis estimating more than $50 billion in annual savings from improved heart failure care alone. (STAT News, h/t Haider Warraich)
The FDA issued a Request for Information exploring a new contracting model designed to work more directly with venture-backed companies developing innovative technologies. The agency is seeking input from venture capital firms on a structure that would allow qualified funds’ portfolio companies to compete for FDA task orders, bypassing traditional intermediaries that often favor labor-based contracts over scalable products. FDA leadership framed the move as a way to accelerate access to innovation across areas like AI, biotech, medical devices, and regulatory technology, while addressing long-standing barriers for early-stage companies. Responses to the RFI were due January 18, 2026 (FDA.gov, h/t Sandeep Patel)
The FDA announced major changes to its oversight of wearables and AI-enabled digital health tools, easing regulation for certain clinical decision support software. Speaking at CES, FDA Commissioner Marty Makary said the agency wants to move at “Silicon Valley speed” and create a more investor-friendly environment, allowing some AI tools to enter clinical workflows without prior FDA review if they meet exemption criteria. (STAT, Latham, h/t Sina Fateh)
The U.S. Government Accountability Office released a new report calling for urgent action to fix persistent weaknesses in the U.S. organ transplant system. The January 22 report finds that U.S. Department of Health and Human Services has identified major risks in organ allocation, oversight, and IT reliability but has not yet laid out a detailed, time-bound plan to implement reforms under its OPTN modernization initiative. GAO also raised concerns about the system’s longtime contractor offering optional supplemental services to transplant programs, generating $9.6 million in fees in FY2024, without HHS formally assessing the risks or transparency of those arrangements. The watchdog recommended that HHS define clear milestones for modernization, evaluate contractor conflicts, and strengthen interagency coordination; HHS agreed with all three recommendations, signaling that significant changes to transplant governance may now be imminent.
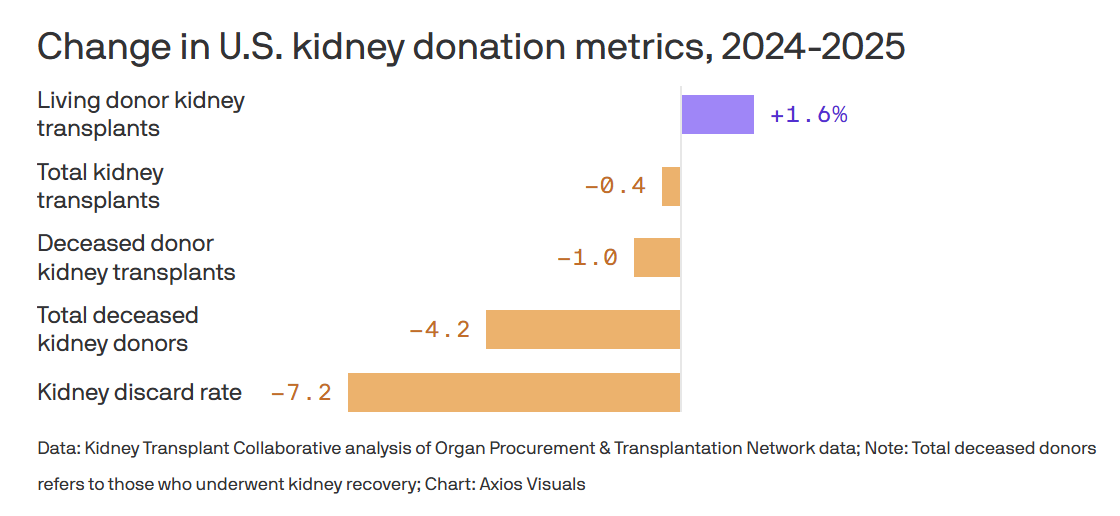
CMS selected Nevada Donor Network to take over organ procurement services in South Florida after decertifying the University of Miami–affiliated Life Alliance Organ Recovery Agency for failing to meet federal performance standards. The transition will be overseen by CMS to ensure uninterrupted donation and transplant services for a region serving roughly 7 million people. This reflects intensified federal pressure to improve accountability across the transplant system. The move comes as deceased kidney donation declined for the first time in more than a decade, following heightened scrutiny and reports that have led some Americans to remove themselves from donor registries.
Research
ARTIFICIAL INTELLIGENCE
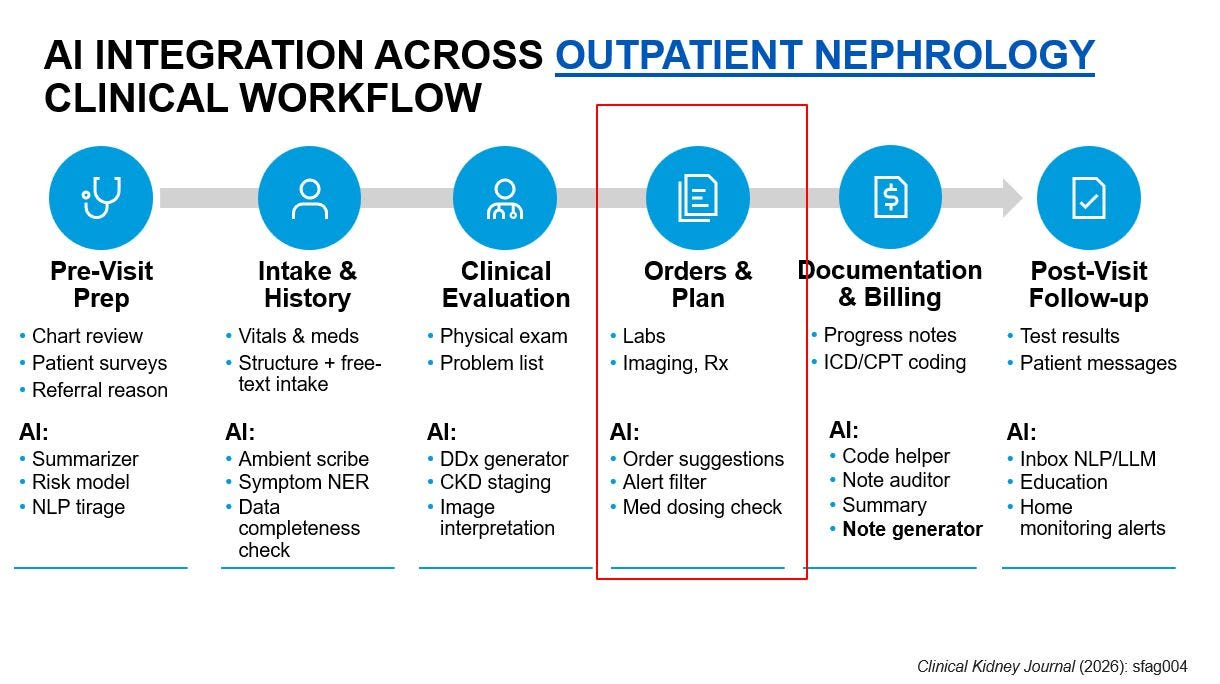
A state-of-the-art review in Clinical Kidney Journal outlines how artificial intelligence is beginning to reshape nephrology across AKI, CKD, dialysis, and transplantation. The authors highlight promising use cases, from early AKI prediction and CKD risk stratification to dialysis optimization and transplant tools like the iBox system, which has already gained regulatory recognition. The roadmap emphasizes that the next frontier is not autonomous care, but clinically integrated, workflow-aware decision support that pairs strong validation with thoughtful implementation to deliver more precise and equitable kidney care. (H/t Wisit Cheungpasitporn, Joe Ix)
A feature in the latest issue of Kidney News argues that artificial intelligence has moved from experimentation to core infrastructure in kidney care, influencing clinical decisions, workflows, and patient engagement. The article highlights a new statement from the American Society of Nephrology AI Workgroup outlining principles for responsible AI use, including patient benefit, clinician oversight, and transparency. Paired with priorities from the KDIGO Controversies Conference, the piece frames the next phase of nephrology AI as a shift from building algorithms to integrating them responsibly at scale to support precision, trust, and equity in kidney care. (H/t Wisit Cheungpasitporn)
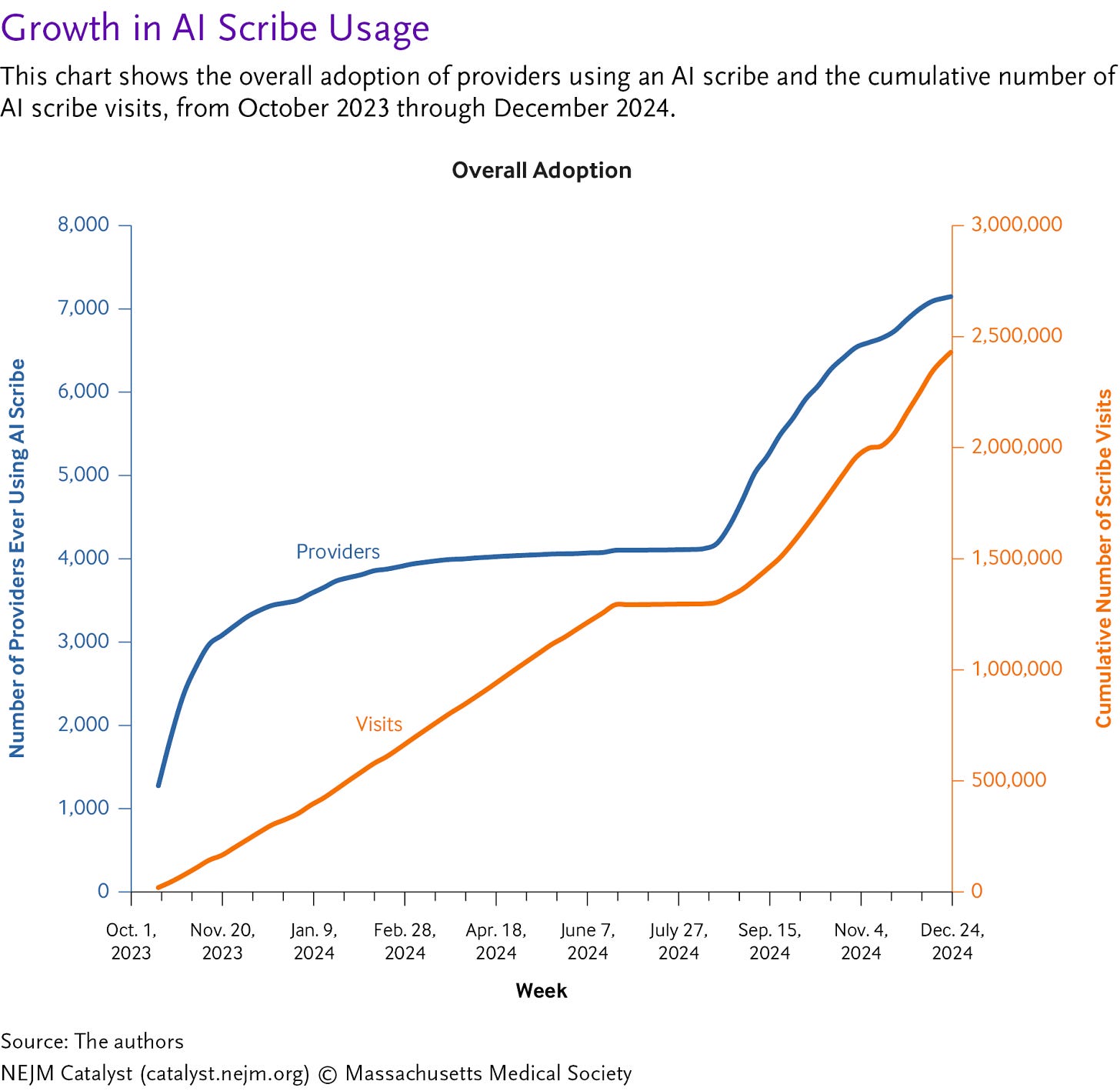
Figure: AI Scribe Adoption Over 1-Year Period
An editorial in NEJM AI takes a clear-eyed look at the current limits of AI scribes, arguing they are not yet the productivity breakthrough many hope for. Drawing on two randomized trials, the authors show modest reductions in documentation time and mixed effects on burnout, with real-world gains often amounting to less than a minute per visit. The piece argues that meaningful productivity gains will likely come only as AI scribes expand beyond note-taking into downstream tasks like orders, prior authorizations, and billing. Until then, AI scribes may help at the margins, but they are not yet a transformative fix for clinician workload. (H/t Karandeep Singh)
A narrative review in Journal of General Internal Medicine critiques the rapid growth of wellness testing, arguing that direct to consumer genomics, broad lab panels, wearables, and whole body imaging have far outpaced the evidence. The authors estimate these low value diagnostics drive more than $100B in annual downstream U.S. healthcare costs while exposing patients to false positives, anxiety, and bias. Instead, they propose a three tier precision health framework that uses AI to guide high sensitivity screening, targeted confirmation, and precision interventions based on pre test probability, with the goal of reducing waste and refocusing prevention on proven care. (H/t Pranav Rajpurkar)
An editorial in Nephrology Dialysis Transplantation discusses emerging preclinical evidence that CAR T-cell therapy could directly target kidney fibrosis, a core driver of CKD progression. Building on work by Zhao et al., the authors highlight a PDGFRβ-targeted CAR T approach that reduced fibrosis and preserved GFR in animal models, including a novel in vivo strategy using lipid nanoparticle–delivered mRNA to generate transient CAR T cells. If translated to humans, this off-the-shelf, non-oncologic CAR T platform could challenge the long-held view of CKD as only slowly modifiable and open the door to fibrosis-directed therapies alongside existing nephroprotective drugs. (H/t Lars Koch)
CLINICAL RESEARCH
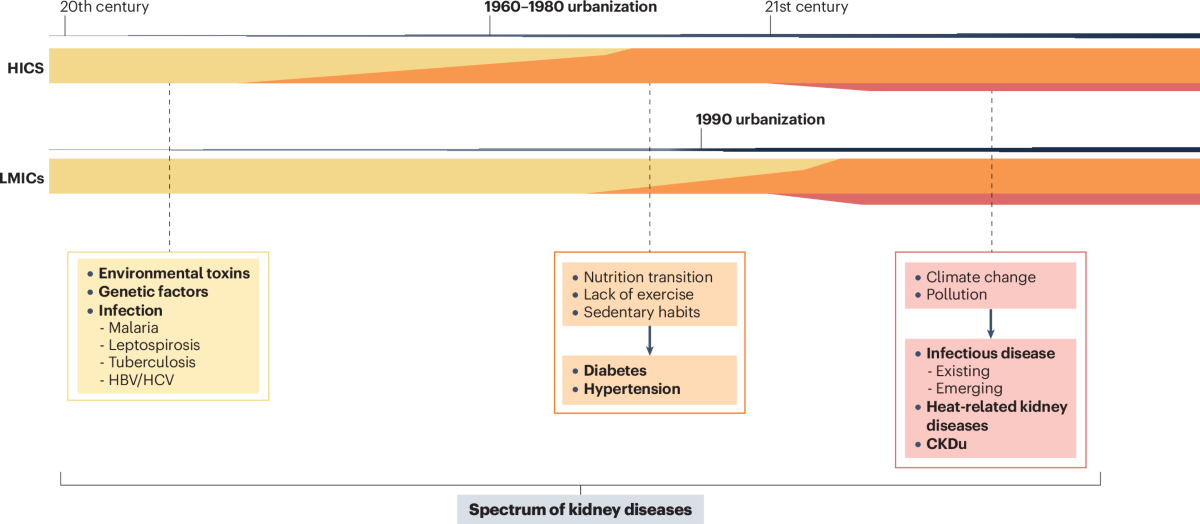
A review in Nature Reviews Nephrology argues that forces like urbanization, climate change, pollution, and shifting lifestyles are increasingly driving CKD and AKI risk, particularly in low- and middle-income countries where health systems are least equipped to respond. The authors call for integrating social determinants into kidney research, policy, and care models to better predict disease trends and reduce global inequities. (H/t Augusto Cesar)
A new call to action in The American Journal of Preventive Cardiology urges routine CKD screening as a core strategy to reduce cardiovascular risk and improve outcomes. The paper emphasizes dual screening with eGFR and UACR, noting that most people with CKD remain undiagnosed and are more likely to die from cardiovascular disease than progress to kidney failure. While this message is well established in nephrology, the authors argue that cardiologists and primary care clinicians must now take a leading role in early detection and implementation of guideline-directed therapies. The piece marks an important shift, signaling growing recognition within cardiology that kidney disease is central to cardiovascular prevention, not peripheral. (H/t Erin Michos)
A synopsis in Annals of Internal Medicine outlines the updated 2025 joint CKD guideline from the U.S. Department of Veterans Affairs and the U.S. Department of Defense, signaling a continued shift of CKD care into primary care. The guidance emphasizes earlier diagnosis, team-based management, clearer referral pathways, and broader use of evidence-based therapies to reduce cardiovascular risk, slow progression, and improve survival. The update reflects how rapidly CKD management has evolved and reinforces the central role of primary care in delivering modern kidney care.
A new study in Circulation shows that diseased kidneys release circulating extracellular vesicles carrying toxic microRNAs that travel to the heart and impair cardiac function, offering a mechanistic explanation for why cardiovascular disease drives mortality in CKD. In animal models, blocking these vesicles improved heart function, pointing to new opportunities for biomarkers and targeted therapies to identify and protect high-risk patients earlier.
An expanding wave of phase 3 trial data is rapidly reshaping IgA nephropathy care, with multiple immunosuppressive and non-immunosuppressive therapies showing meaningful proteinuria reduction across different mechanisms. Evidence suggests additive benefits when combining RAAS, SGLT2, endothelin blockade, and newer agents such as APRIL/BAFF inhibitors, but key questions remain around optimal sequencing, upfront combination therapy, and treatment of high-risk groups excluded from trials. Future progress will depend on coordinated trials, real-world data, and better phenotyping to match patients to the right therapies earlier. (Kidney News, h/t Ayman Al Jurdi)
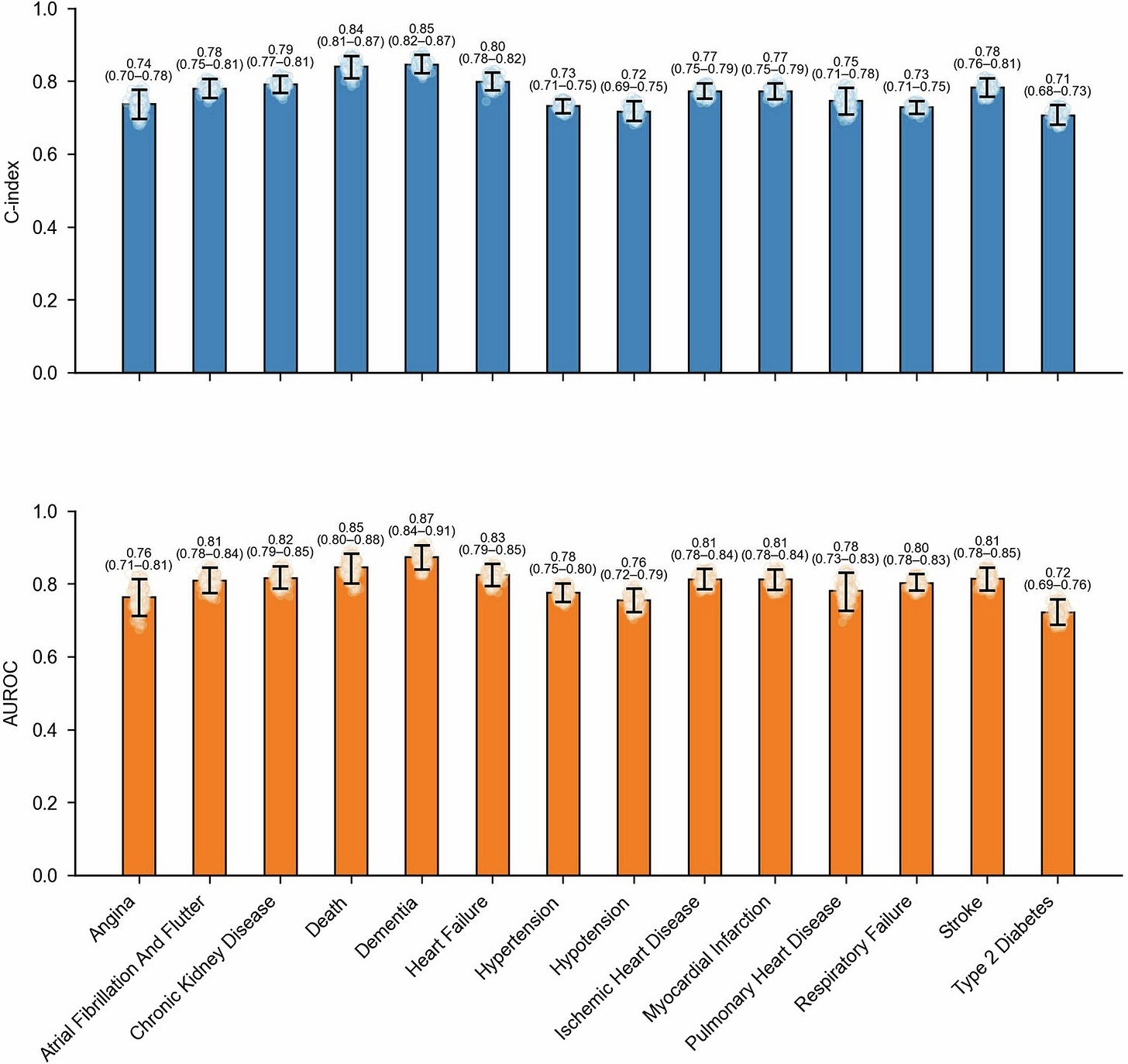
Figure: SleepFM Study: Performance across clinically relevant diseases evaluated on Stanford data (n=5019)
A paper in Nature Medicine introduces SleepFM, a large multimodal AI model trained on more than 585,000 hours of polysomnography data from 65,000 people to predict future disease risk from a single night of sleep. The model accurately predicted 130 conditions years before diagnosis, including chronic kidney disease, heart failure, stroke, dementia, and all-cause mortality, outperforming traditional demographic risk factors. By learning a unified representation of brain, heart, muscle, and breathing signals, the study positions sleep as a powerful and underused biomarker for early disease detection, including kidney disease, at population scale. (H/t James Zou)
A study in Kidney International used data from the Mass General Brigham Biobank to better understand the genetic causes of nephrotic syndrome in adults. The researchers found that 17% of patients had a clear genetic form of disease, even though many were labeled as having “secondary FSGS,” a diagnosis that often leads clinicians to skip genetic testing. The study also identified variants in the MEFV gene as a previously underrecognized risk factor for FSGS, even in patients without classic signs of Familial Mediterranean Fever. Overall, the work supports broader genetic testing in adult kidney disease and points to more personalized treatment approaches. (H/t Matt Sampson)
A behind-the-paper look in Communications Biology explains why kidney scarring in diabetes is so difficult to reverse, even with good glucose control. The authors describe how a small regulatory RNA, miR-299a-5p, becomes elevated in diabetic kidneys and suppresses the organ’s natural antifibrotic defenses, driving ongoing scar formation. In animal models, blocking this microRNA restored protective pathways and improved kidney function, suggesting that durable RNA-level changes may lock diabetic kidney disease into a self-perpetuating course. (H/t Kennedy Nmecha)
A recent case report highlights side effects with obinutuzumab, a newer anti CD20 therapy increasingly used for difficult to treat immune mediated glomerular diseases. While the drug offers deeper and more durable B cell depletion than rituximab, the authors describe three patients who developed severe hematologic toxicities, including grade 4 neutropenia and persistent anemia, even when used as monotherapy. The findings underscore the need for careful patient selection, close monitoring, and a clearer understanding of risk tradeoffs as newer immune therapies move into routine kidney care.
MARKET TRENDS
The 2025 HSBC Venture Healthcare Report finds that U.S. healthcare venture capital began to recover from the recent downturn, with investment rebounding late in the year, driven largely by AI-enabled technology rounds and increased M&A activity. While overall deal volume remained constrained, capital concentrated into larger, later-stage financings, particularly mega rounds in biopharma and outsized raises in medtech and healthtech. Looking ahead, HSBC projects modest growth in 2026, with total healthcare venture investment reaching $65–70B, supported by renewed IPO activity, stronger M&A, and growing participation from crossover investors and corporates. The report suggests the next cycle will favor scale, capital efficiency, and platforms that combine clinical impact with durable business models. (H/t Jon Norris)
Carta data shared by Peter Walker shows that Q4 2025 was the most active quarter for startup M&A on record, with 232 acquisitions, up 60% from Q4 2023. Nearly half of deals involved pre-seed and seed-stage companies, reflecting a surge in acquihires and competition for AI talent, while a record number of acquired companies had raised more than $50M. More than 40% of acquisitions involved AI startups, highlighting how quickly AI-native capabilities are becoming core to corporate strategy, even as many deals fail to generate returns for common shareholders.
A new report from Hospitalogy offers a detailed look at the current and emerging state of U.S. health systems heading into 2026. The report examines macro trends shaping hospital strategy, including automation and AI adoption, physician employment pressures, specialty drug economics, and shifting nonprofit and for profit performance benchmarks. It also includes health system case studies, financial tear sheets, and analysis of where AI can meaningfully drive margin expansion in areas like prior authorization, denials management, and revenue cycle operations. (H/t Blake Madden)
Caroline Pearson of the Peterson Health Technology Institute argues that more than $50B invested in digital health has delivered mixed results, highlighting the need for stronger evidence and accountability. After reviewing dozens of tools across healthcare, PHTI finds that technology works best when it scales proven care models, replaces existing services instead of adding cost, and targets the right patients at the right time, while many behavior-change apps have fallen short. I believe this piece reinforces a clear through line from the growing cardio-kidney-metabolic care paradigm: the next phase of health tech must be defined by measurable outcomes, real-world validation, and performance-based adoption, not hype. (H/t Caroline Pearson)
Community
In his first editorial as president of the American Society of Nephrology, Samir Parikh frames Hope in Nephrology as both a personal reflection and a call to action, alongside ASN’s new 2026–2027 Strategic Plan (see below). He highlights progress made in 2025 across research funding, prevention, transplant policy, and workforce support, while emphasizing that hope must translate into execution, equity, and fair compensation for nephrologists. The piece positions optimism not as sentiment, but as a responsibility to patients, trainees, and the future of kidney care. (H/t Samir Parikh)
A webinar hosted by HMA and Leavitt Partners breaks down the decade-long, voluntary ACCESS model, which creates a new Medicare payment pathway for digital health, remote monitoring, behavioral support, and co-managed chronic care. For technology companies and value-based care operators, this is one of the clearest signals yet of how CMS plans to operationalize innovation at scale and what applicants should be doing now to prepare for the upcoming RFA (H/t Ryan Howells and Kate de Lisle).
National Kidney Foundation highlights 5 FDA approvals in 2025 targeting specific kidney disease pathways, from diabetes-related CKD to rare immune-driven disorders like IgAN and C3G. Newly approved therapies span GLP-1s, complement inhibitors, monoclonal antibodies, and endothelin receptor antagonists. It shows kidney care is finally moving beyond one-size-fits-all treatment toward precision therapies (NKF).
A major milestone for pediatric kidney care: the first patient has been enrolled in the first global prospective pediatric CKD trial evaluating SGLT2 inhibitors. The study builds on a 2023 convening led by the Kidney Health Initiative and NephCure to address trial design, safety, and regulatory gaps. The launch marks a concrete step toward evidence-based therapies for children with CKD and shows how coordinated leadership can translate long-standing needs into action. (H/t Barbara Gillespie, Fortrea)
An article by Rob Blaser and Rebecca Schmidt in Healio argues that kidney policy advocacy is more important than ever as payment models and care delivery continue to shift. It highlights the long-standing role of the Renal Physicians Association in shaping kidney reimbursement and representing nephrology within the AMA, with a 2026 agenda focused on payment reform, value-based care, living donation, prior authorization, and telehealth. The piece makes the case that sustained advocacy is essential to protect patients and enable modern kidney care as the system evolves. (H/t Rob Blaser)
Emory Healthcare set a new U.S. record in 2025 with 591 adult kidney transplants, the most ever by a single program in one year. Emory led the nation in both living and deceased donor transplants and performed more kidney transplants in African American patients than any other center, expanding access despite a national decline in organ donation.
AbsoluteCare appointed Alice Wei as Nephrology Medical Director as it launches a statewide Medicaid CKD initiative in Ohio. Beginning in 2026, the program will deliver whole-person, wraparound kidney care to nearly 10,000 high-need members across urban and rural communities. AbsoluteCare reported a 40% reduction in hospital dialysis starts and higher use of guideline-directed CKD meds. Congrats Alice!
A new Kidney International editorial describes how a Fiji-led collaboration helped catalyze kidney supportive care in a resource-limited setting, where dialysis access is constrained and symptom burden is high. The piece lays out culturally grounded principles, workforce realities, and a phased pathway to embed supportive and conservative kidney care into routine practice, emphasizing nonabandonment, community engagement, and pragmatic capacity building across the Pacific. (H/t Shilpa Jesudason)
Venova Medical announced successful initial patient enrollments in the VENOS-3 Pivotal Study evaluating its Velocity® pAVF System, led by Dr. Rishi Razdan and the team at Azura Vascular Care in Jacksonville, Florida. The milestone marks early progress in Venova’s effort to advance percutaneous AV fistula creation as a less invasive option for hemodialysis vascular access.
In an op-ed in MedPage Today, Macey Levan argues that U.S. transplantation has outgrown its current federal governance and needs a dedicated bureau focused solely on transplant policy and oversight. She warns that limited transparency and stakeholder engagement in OPTN modernization risks weakening a system that depends on clinician and patient participation. She believes a standalone transplant bureau would strengthen accountability, modernize infrastructure, and keep patients and professionals at the center of reform.
In an internal analysis, Monogram Health reports that its multispecialty, home-based model for polychronic patients is associated with 44% fewer hospital admissions, 52% fewer ED visits, and $160M in partner cost savings in 2024, rising to $375M in 2025. The results reinforce growing evidence that coordinated, whole-person care—especially for kidney and cardio-metabolic populations—can improve outcomes while materially bending the cost curve. (H/t Shammi Gupta)
Bobby Lawson wrote a great post about how care delivery often breaks at the contract level. A sharp take on why payer–provider agreements fail not in negotiation, but in design: built on assumptions about workflows, incentives, and economics that don’t hold up in real-world care delivery, leading to margin pressure, operational drag, and patient access friction.
We’d love to hear from you. What questions and comments do you have from this month’s recap? What stood out to you, and what else should we know?
Events Calendar
ASTS Winter Symposium (Jan 22-25, Phoenix, AZ)
Mayo Clinic Clinician Update (Feb 13-15, Scottsdale, AZ)
AHIP: AI In Kidney Care (Feb 26 at 12pm ET)
WCN (Mar 28-31, Yokohama, Japan)
RPA Annual Meeting (Apr 16-19, Atlanta, GA)
Columbia CKM: Evolving Frontiers (May 1, Virtual)
NephCure Support Groups (Ongoing)
Jobs
Nephrologist (Miami, FL) — Kaüna (Say hello!)
Comp. Biologist Post-Doc — Harvard Med
Director, Rural Health Program — State of Maine
Assoc. Director, Patient Marketing — Vertex
Associate, Healthcare Incubations — Primary VP
Lead Director, Pop Health — CVS Health
Analyst, Clinical Transformation — DaVita
Data Fellow — Rock Health
VP, Managing Director — GEHA Health Ventures
Clinical Field Specialist — MediBeacon
Director, Network Strategy — Strive Health
…plus hundreds more at jobs.signalsfs.com
Work with us
Signals Group is expanding to support the growth of this community. Whether you’re looking to increase awareness for your work, prepare for your next milestone, or looking to enter a new market, we’d love to hear from you. We support mission-aligned organizations advancing kidney health.
Learn — Discover hundreds of articles & interviews in our Data Room
Share — See how Signals can help you reach your next milestones
Sponsor — See why our 2026 sponsorships are filling up fast
This audio summary may include variations in pronunciation and is intended for informational purposes only. For complete accuracy and source attribution, please refer directly to the original written materials and cited sources. Always consult trusted references when interpreting medical or scientific content.
![Signals From [Space]](https://substackcdn.com/image/fetch/$s_!IXc-!,w_40,h_40,c_fill,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F9f7142a0-6602-495d-ab65-0e4c98cc67d4_450x450.png)
![Signals From [Space]](https://substackcdn.com/image/fetch/$s_!lBsj!,e_trim:10:white/e_trim:10:transparent/h_48,c_limit,f_auto,q_auto:good,fl_progressive:steep/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F0e0f61bc-e3f5-4f03-9c6e-5ca5da1fa095_1848x352.png)
![Signals From [Space]](https://substackcdn.com/image/fetch/$s_!NnOt!,w_144,h_144,c_fill,f_auto,q_auto:good,fl_progressive:steep,g_auto/https%3A%2F%2Fsubstack-post-media.s3.amazonaws.com%2Fpublic%2Fimages%2F688fc47b-7202-4a2e-b4f4-fea2b047ab1b_1500x1500.png)